- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
New and Emerging Therapies for Retinal Diseases презентация
Содержание
- 1. New and Emerging Therapies for Retinal Diseases
- 2. Disclosures No financial interests to disclose Dr. Chiu has been a consultant to Alimera Sciences
- 3. Outline I. Diabetic Macular Edema A. Anti-VEGF
- 4. Diabetic macular edema 26 million Americans with
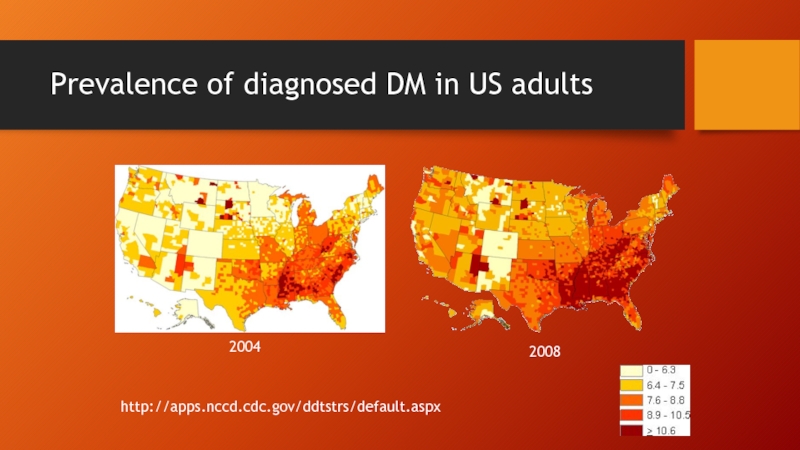
- 5. Prevalence of diagnosed DM in US adults 2004 2008 http://apps.nccd.cdc.gov/ddtstrs/default.aspx
- 6. Prevalence of diagnosed DM in Maryland adults
- 7. Diabetic macular edema “Standard of care” Focal/grid
- 8. Anti-VEGFS
- 9. Ranibizumab(Lucentis) for DME FDA approved for diabetic
- 10. RIDE and RISE studies 36 month Phase
- 11. RISE RIDE Sham group received Lucentis from mo 24-36
- 12. RIDE/RISE 24 month data
- 13. RIDE/RISE Not so impressive findings 23% treated
- 14. In the real world… Treatment burden Cost
- 15. Aflibercept (Eylea) FDA approved for diabetic macular
- 16. VIVID (EU and Japan)/VISTA (US) studies Phase
- 17. VISTA VIVID
- 18. Bevacizumab (Avastin) $42 per dose for intravitreal
- 19. BOLT study 24 month prospective study for
- 20. DRCR.net Protocol T NIH sponsored studying comparing
- 21. Corticosteroids
- 22. Corticosteroids Role of inflammatory cytokines BESIDES VEGF
- 23. Fluocinolone (Iluvien) Alimera Sciences Non-biodegradable implant, releases
- 24. FAME study Two 3 year Phase III
- 25. Dexamethasone (Ozurdex) Injectable biodegradable intravitreal implant, 3-6
- 26. MEAD study 3 year phase III study
- 27. MEAD study – pseudophakic/anticipated cataract surgery participants
- 28. Corticosteroids Consider using if/when DME patients are:
- 29. AMD How far we have come What
- 30. AMD – How far have we come
- 31. AMD – How far have we come
- 32. AMD – How far we have come
- 33. AMD – What do we do (with
- 34. IVAN (“British CATT”) 2 key questions
- 35. AMD- What do we do (with what
- 36. AMD - Where are we going Sustained release Quest for the “Everlasting Gobstopper”
- 37. AMD – Where are we going Encapsulated
- 38. Encapsulated cell technology (ECT)
- 39. AMD – Where are we going Wet
- 40. AMD – Where are we going Nanostructured
- 41. Nanostructured Tethadur
- 42. AMD – Where are we going Port
- 43. AMD – Where are we going MicroPump
- 44. AMD – Where are we going Beyond
- 45. Anti-platelet derived growth factor therapy The problem
- 46. Anti-PDGFs
- 47. Platelet derived growth factor (PDGF) What’s in
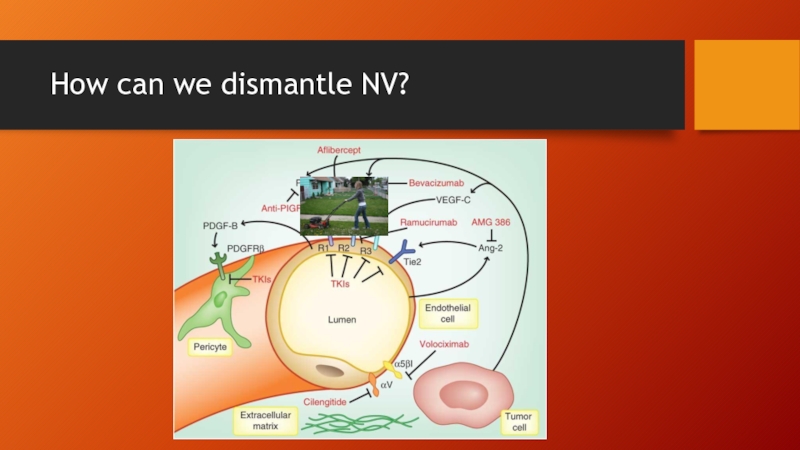
- 48. How can we dismantle NV?
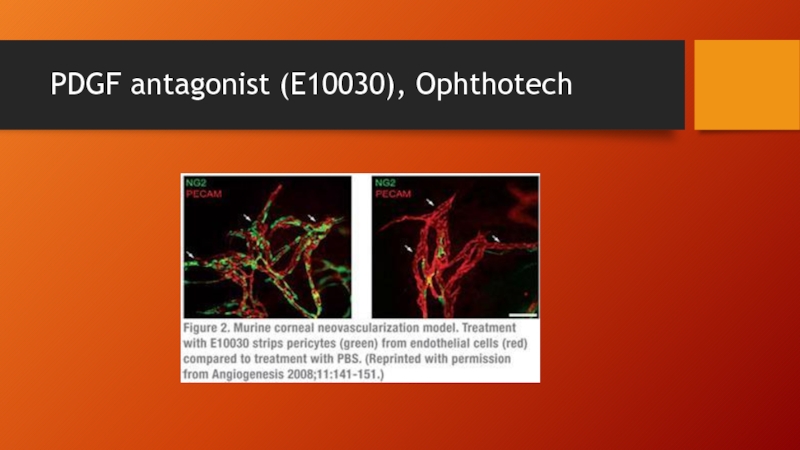
- 49. PDGF antagonist (E10030), Ophthotech
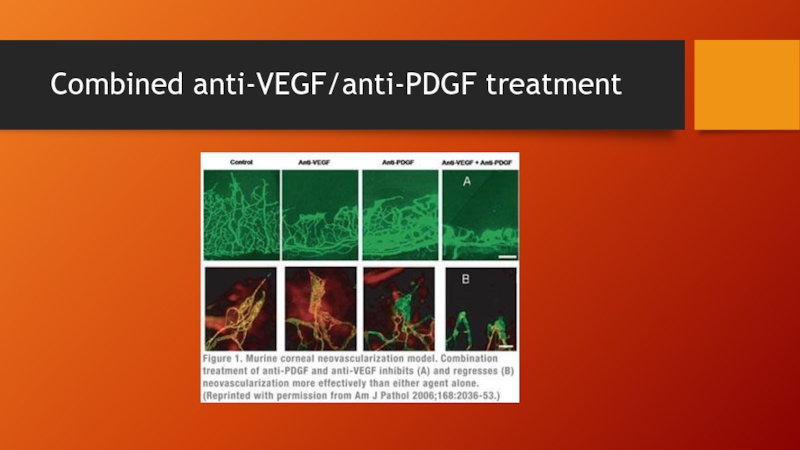
- 50. Combined anti-VEGF/anti-PDGF treatment
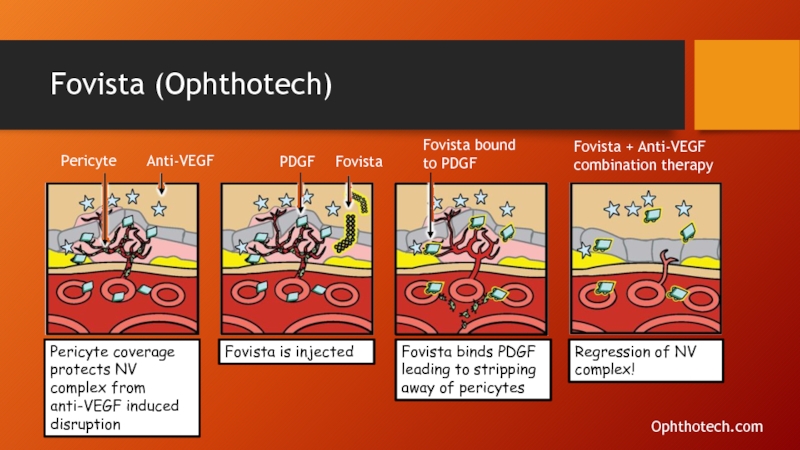
- 51. Fovista (Ophthotech) Pericyte Anti-VEGF PDGF Fovista Fovista
- 52. Fovista (Ophthotech) Phase 2 study 24 week
- 53. Anti-PDGF Also being developed by: Neurotech using
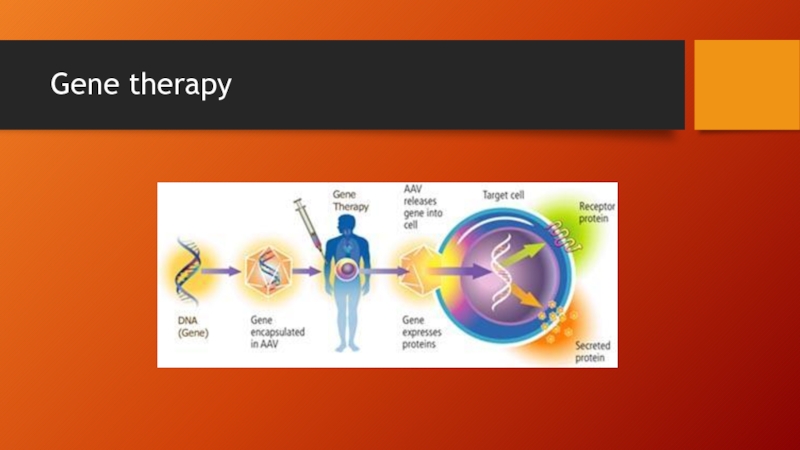
- 54. Gene Therapy
- 55. Gene therapy Therapy in which genetic material
- 56. Gene therapy Desirable features of viral vectors
- 57. Gene therapy
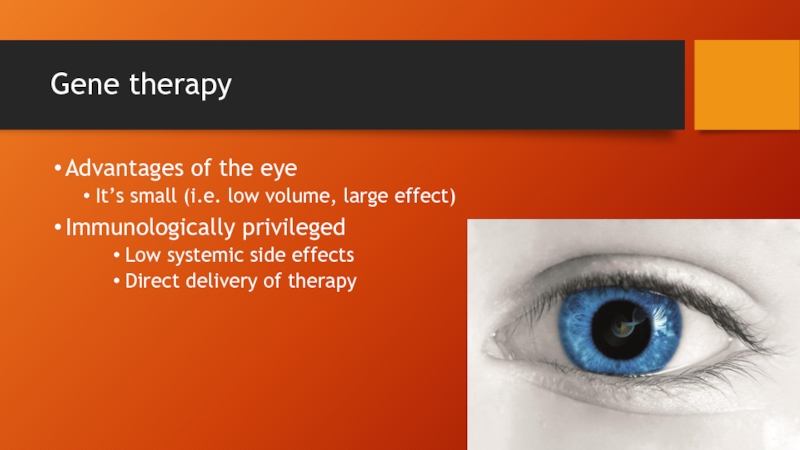
- 58. Gene therapy Advantages of the eye It’s
- 59. Ongoing retinal gene therapy trials Wet AMD
- 60. AAV2-sFlt01 Intravitreal Therapy (Genzyme/Sanofi) Induces expression
- 61. AAV2-sFlt01 Subretinal therapy (Avalanche Biotech) Phase 1
- 62. AAV2-sFlt01 http://permalink.fliqz.com/aspx/permalink.aspx?at=2315379b3136443fa353b26aaf11d582&a=375d1defb6aa477988c6708adf47c1e7
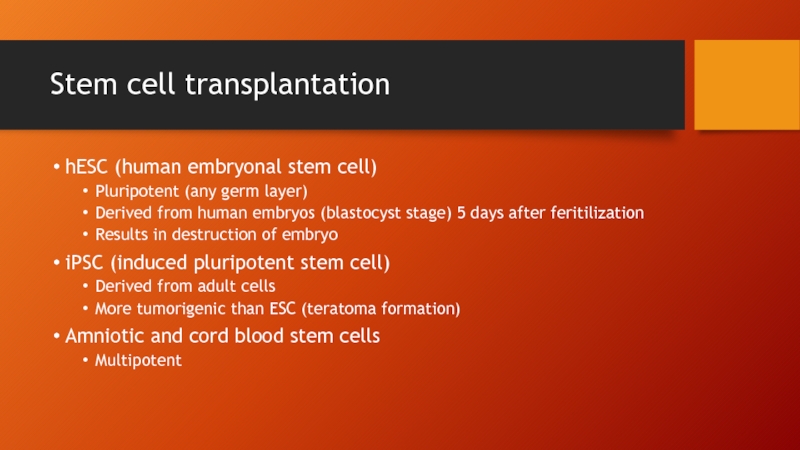
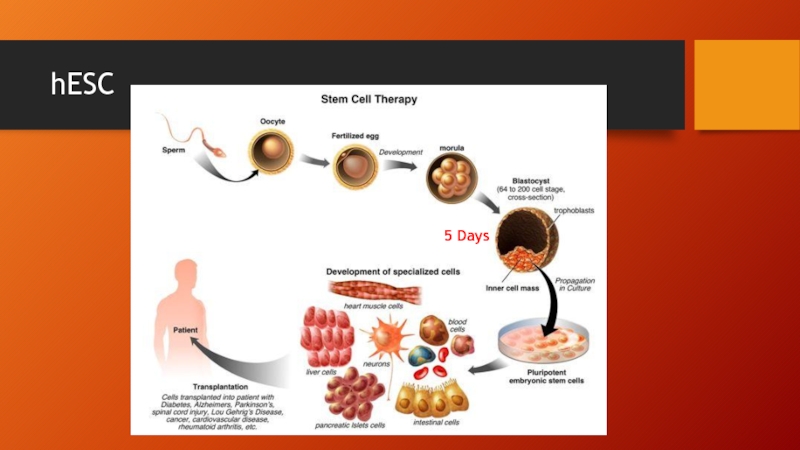
- 63. Stem cell transplantation hESC (human embryonal stem
- 64. hESC 5 Days
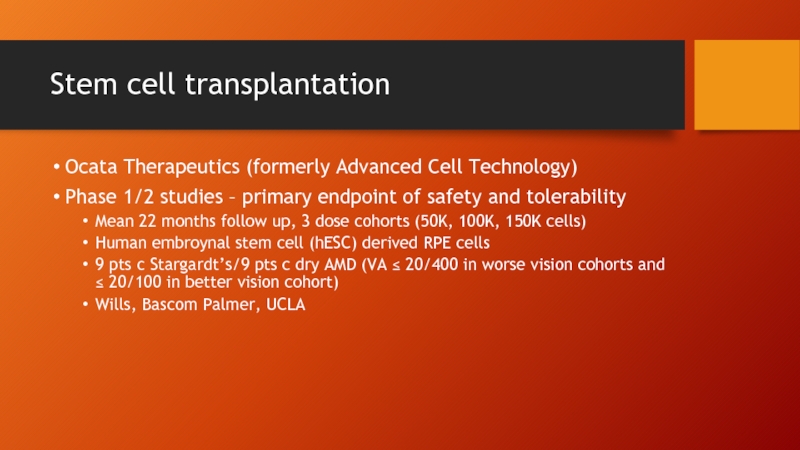
- 65. Stem cell transplantation Ocata Therapeutics (formerly Advanced
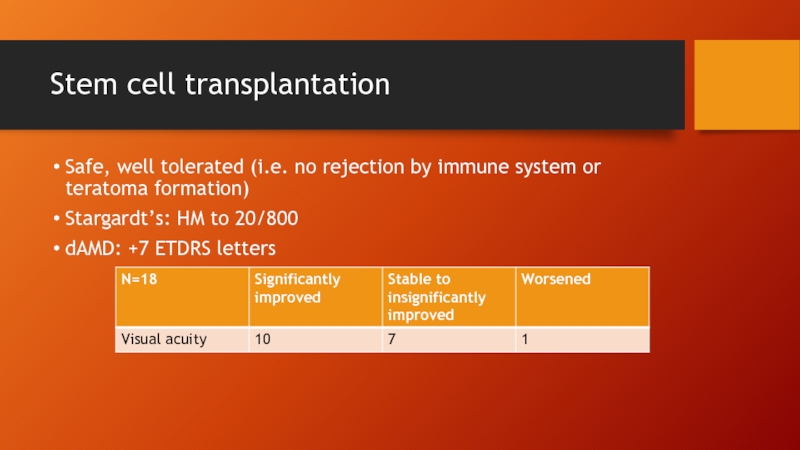
- 66. Stem cell transplantation Safe, well tolerated (i.e.
- 67. Complement cascade inhibition Geographic atrophy Loss of
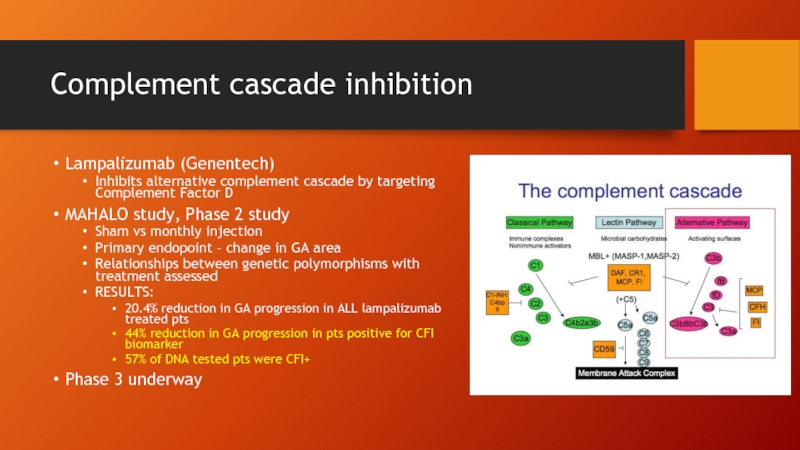
- 68. Complement cascade inhibition Lampalizumab (Genentech) Inhibits alternative
- 70. Topical therapy
- 71. Squalamine Originally derived from dogfish shark liver,
- 72. Squalamine eye drops Ohr Pharmaceutical Rapid uptake
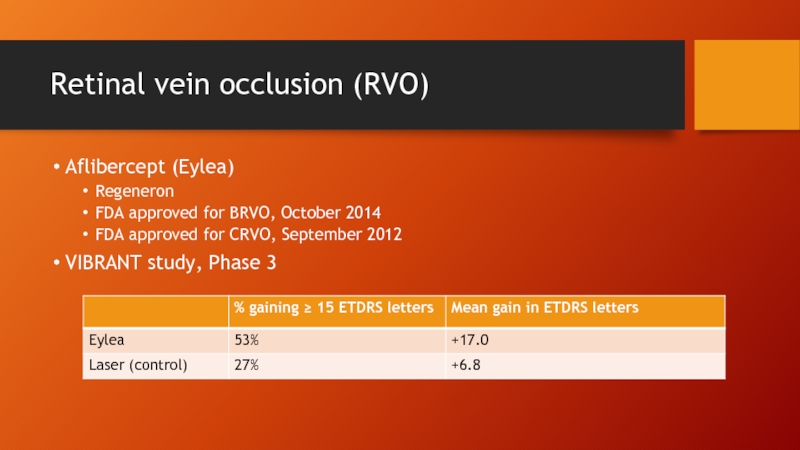
- 73. Retinal vein occlusion (RVO) Aflibercept (Eylea) Regeneron
- 74. Retinal vein occlusion (RVO) Similar results for
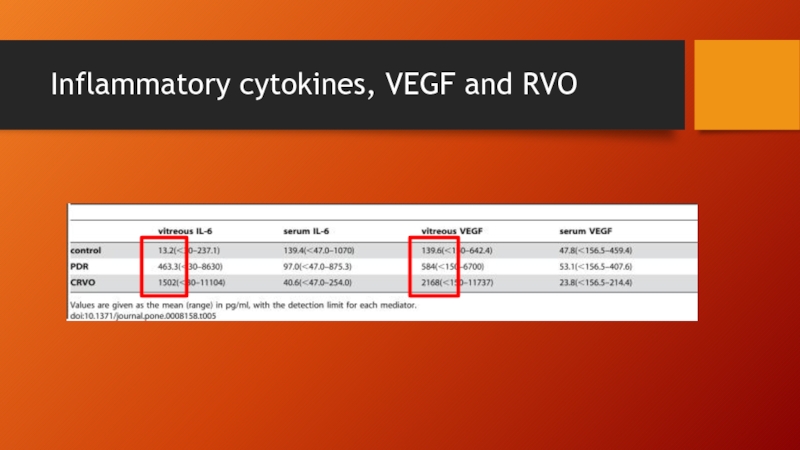
- 75. Inflammatory cytokines, VEGF and RVO
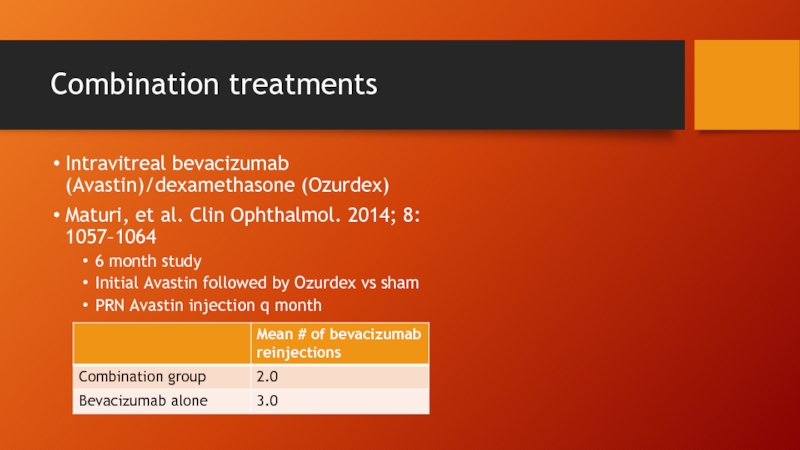
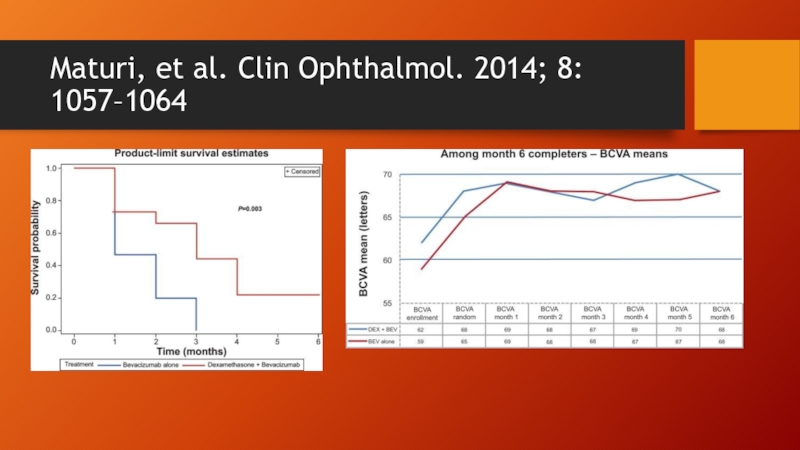
- 76. Combination treatments Intravitreal bevacizumab (Avastin)/dexamethasone (Ozurdex) Maturi,
- 77. Maturi, et al. Clin Ophthalmol. 2014; 8: 1057–1064
- 78. Conclusions Most patients with wet AMD, DME,
- 79. DME 20/160 20/60 20/30 20/30
- 80. 67 y.o. male with
- 81. CRVO 56 year old male with BRVO
- 82. CRVO 46 y.o. male with CRVO Initial
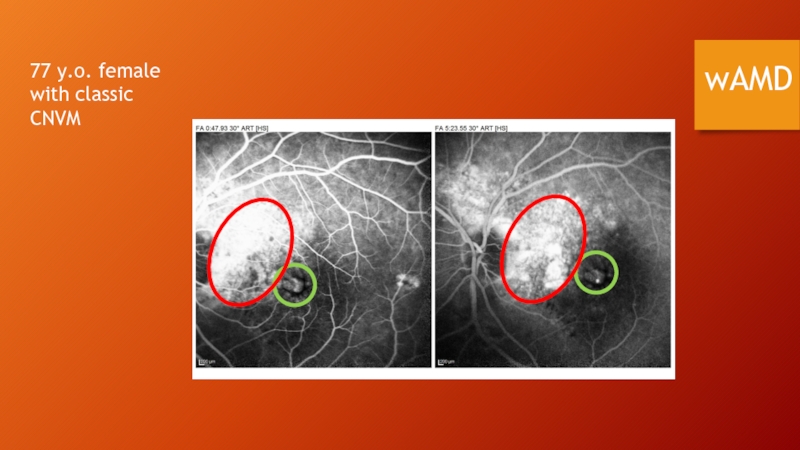
- 83. wAMD 77 y.o. female with classic CNVM
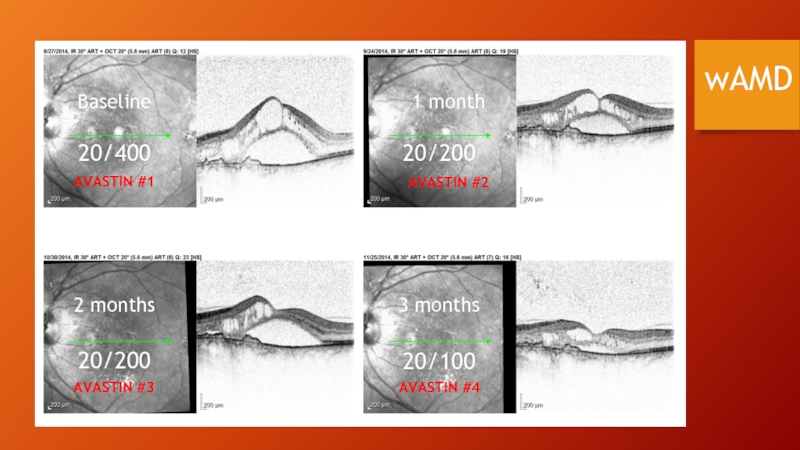
- 84. wAMD Baseline 1 month 2 months 3
- 85. Thank you
Слайд 1New and Emerging Therapies for Retinal Diseases
Toufic Melki, M.D.
Richard Chiu, D.O.
Retina
Locations in Rockville, Arlington, and Georgetown University
Слайд 2Disclosures
No financial interests to disclose
Dr. Chiu has been a consultant to
Слайд 3Outline
I. Diabetic Macular Edema
A. Anti-VEGF therapies
B. Corticosteroids and role of inflammatory
1. Ozurdex
2. Iluvien
II. AMD
A. Sustained delivery devices
B. Platelet derived growth factor inhibition (anti-PDGF therapy)
C. Gene therapy
D. Complement cascade inhibition
E. Squalamine topical therapy
III. RVO
A. Anti-VEGF therapies and differences with DME, wAMD therapy
B. Corticosteroids
IV. Case presentations
Слайд 4Diabetic macular edema
26 million Americans with diabetes
2 million new cases of
19% of current diabetics are undiagnosed
About 10% of patients with DM develop DME in their lifetime
72,000 new cases of DME diagnosed each year
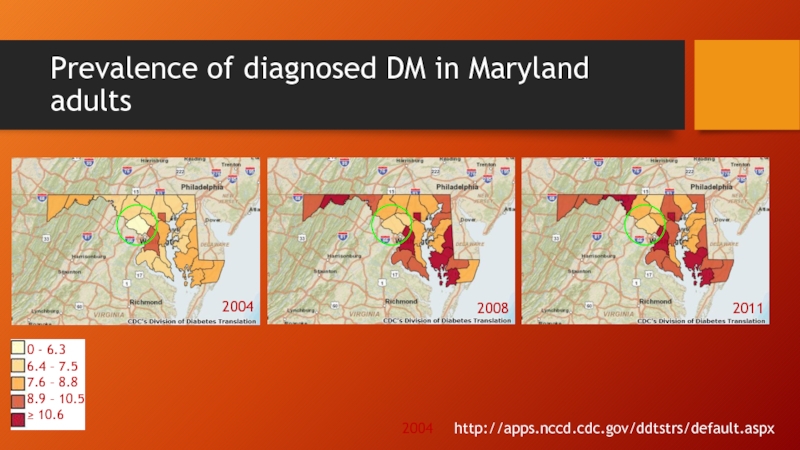
Слайд 6Prevalence of diagnosed DM in Maryland adults
http://apps.nccd.cdc.gov/ddtstrs/default.aspx
2004
2008
2011
2004
0 - 6.3
6.4 – 7.5
7.6
8.9 – 10.5
≥ 10.6
Слайд 7Diabetic macular edema
“Standard of care”
Focal/grid laser
ETDRS
Clinically significant diabetic macular edema
Focal/grid laser
Visual acuity did not improve
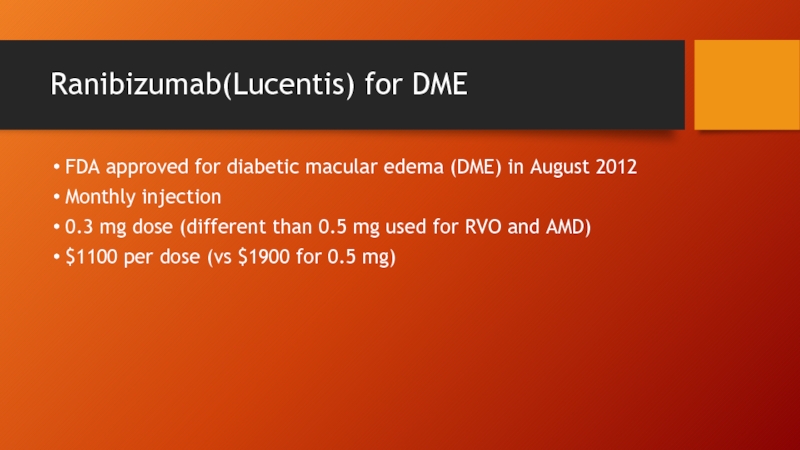
Слайд 9Ranibizumab(Lucentis) for DME
FDA approved for diabetic macular edema (DME) in August
Monthly injection
0.3 mg dose (different than 0.5 mg used for RVO and AMD)
$1100 per dose (vs $1900 for 0.5 mg)
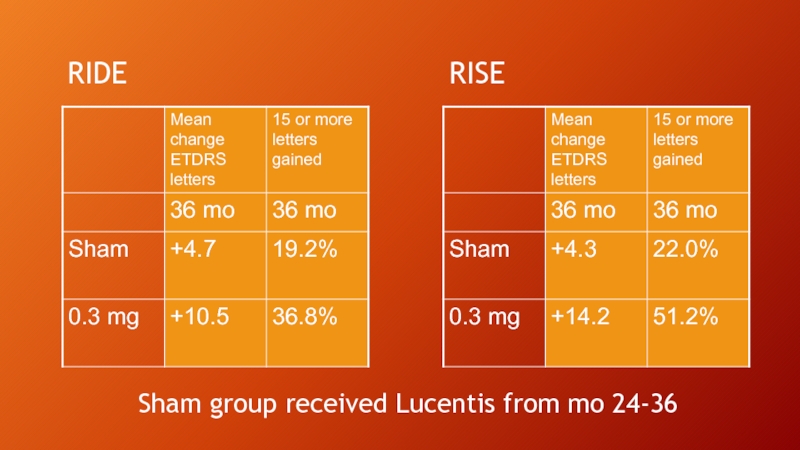
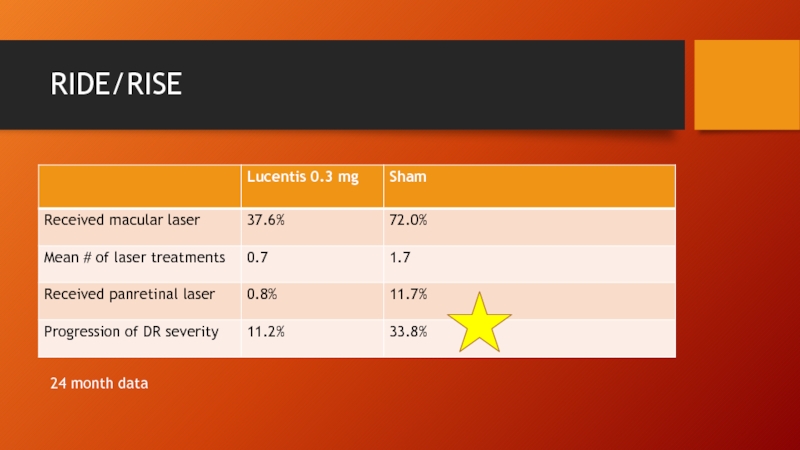
Слайд 10RIDE and RISE studies
36 month Phase III studies for MONTHLY injection
759 patients
VA ranging from 20/40 to 20/320
OCT > 275 microns
Focal laser allowed at 3 months
Слайд 13RIDE/RISE
Not so impressive findings
23% treated patients still had central macular thickness
40% had not achieved VA ≥ 20/40
Слайд 14In the real world…
Treatment burden
Cost
Patient tolerance
Do we really need to inject
DRCR-net Protocol I
Слайд 15Aflibercept (Eylea)
FDA approved for diabetic macular edema (DME) in July 2014
2
Monthly injection x 5 doses, Q 2 months thereafter
$1850 per dose
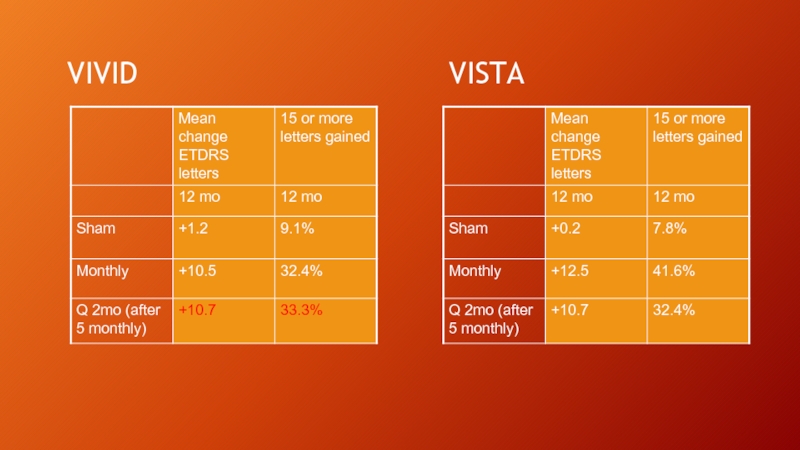
Слайд 16VIVID (EU and Japan)/VISTA (US) studies
Phase III studies comparing Eylea 2.0
VIVID 461 patients, VISTA 466 patients
3 year study
“Rescue” laser given if VA declined >=15 letters at any 1 visit or >= 10 letters in 2 consecutive visits
Слайд 18Bevacizumab (Avastin)
$42 per dose for intravitreal
Cost of treatment for colon CA
Efficacy in wAMD demonstrated to be comparable in CATT study
Слайд 19BOLT study
24 month prospective study for bevacizumab (Avastin)
Mean change in BCVA
Treatment
Laser -0.5 letters
15 letter or more gain
Treatment group 32%
Laser group 4%
Слайд 20DRCR.net Protocol T
NIH sponsored studying comparing 660 patients randomly assigned to
Identical retreatment criteria
AAO 2014
“Teaser” for 12 month data
Eylea treated patients significantly better by 2 ETDRS letters than Lucentis and Avastin treated patients
Eylea treated patients received one fewer injection than Lucentis and Avastin treated patients
Rate of ATE (arteriothromboembolic events) was 2% Eylea, 4% Avastin, 5% Lucentis
Results pending verification prior to publication
Слайд 22Corticosteroids
Role of inflammatory cytokines BESIDES VEGF in DR
IL-6
MCP-1
Anti-VEGF therapy may not
Слайд 23Fluocinolone (Iluvien)
Alimera Sciences
Non-biodegradable implant, releases 0.2 mcg/day
3 year duration of effect
Previously
In use in several E.U. countries
FDA approval September 2014 for DME previously treated with corticosteroids s significant IOP elevation
Available in US 1st quarter 2015, cost $8000 (?)
Слайд 24FAME study
Two 3 year Phase III studies for Iluvien
15 letter or
Treatment group 28.5-32.4%
Sham 13.4%
82-88% of phakic patients received cataract surgery
38-42% required IOP lowering meds
4.8-8.1% required glaucoma surgery
Слайд 25Dexamethasone (Ozurdex)
Injectable biodegradable intravitreal implant, 3-6 mo duration of effect
$1300 per
FDA approved for DME June 2014
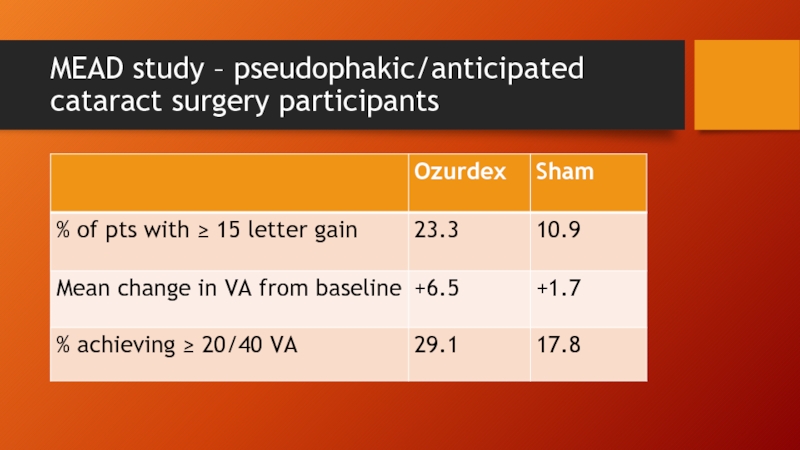
Слайд 26MEAD study
3 year phase III study
Retreatment allowed q 6 mo
Mean 4.1
Adverse outcomes
59% treated patients needed cataract surgery (vs 7% in sham group)
41% required IOP lowering medication
0.7% needed glaucoma filtration surgery
Слайд 28Corticosteroids
Consider using if/when DME patients are:
Pseudophakic or anticipating cataract surgery
Post-vitrectomy
Unable
Poorly responsive or tachyphylactic to anti-VEGF monotherapy
Слайд 30AMD – How far have we come
Bloch SB, et al. Am
Population based observational study, Denmark
Incidence of legal blindness from AMD decreased by 50% from 2000-2010
Most of the decrease occurring after 2006 (i.e. after introduction of anti-VEGF therapy)
Слайд 31AMD – How far have we come
MARINA study
Monthly ranibizumab (Lucentis) injection
+10.7 ETDRS letters
-14.9 letters with sham
-9.8 letters with photodynamic therapy (ANCHOR)
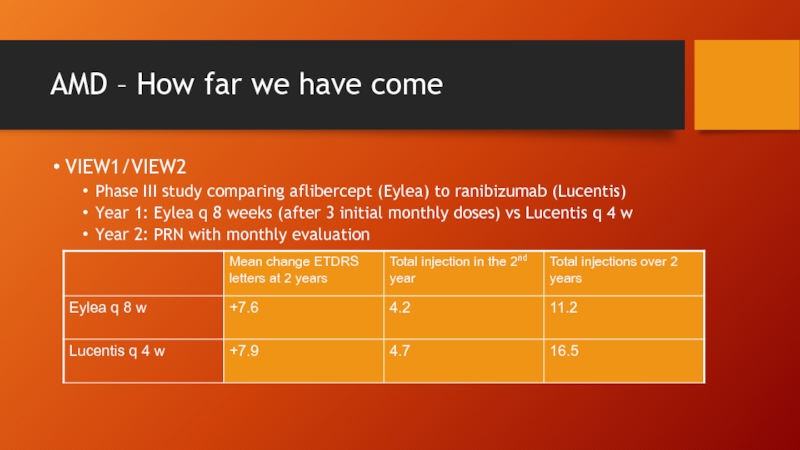
Слайд 32AMD – How far we have come
VIEW1/VIEW2
Phase III study comparing aflibercept
Year 1: Eylea q 8 weeks (after 3 initial monthly doses) vs Lucentis q 4 w
Year 2: PRN with monthly evaluation
Слайд 33AMD – What do we do (with what we have)
CATT
2 key
Comparison of bevacizumab (Avastin) with ranibizumab (Lucentis)
Monthly vs PRN dosing of each
24 month results
Monthly dosing favored over PRN
2.4 letters
Switching to PRN after 1 year of monthly injection resulted in -2.2 letters over continued monthly dosing
VA outcomes slightly favor Lucentis (1.4 letters) but was not statistically significant
Слайд 34IVAN (“British CATT”)
2 key questions
Comparison of bevacizumab (Avastin) with ranibizumab
Continuous (monthly) vs discontinuous (PRN)
24 month results (The Lancet, 382: 1258-1267)
Bevazicumab (-1.37 letters) was “neither inferior nor non-inferior to ranibizumab”
Discontinuous (-1.63 letters) was “neither inferior nor non-inferior to continuous”
AMD – What do we do (with what we have)
Слайд 35AMD- What do we do (with what we have)
HARBOR study
Ranibizumab (Lucentis)
0.5
Monthly vs PRN after 3 monthly doses
Retreatment if OCT demonstrated fluid or if VA decreased by ≥ 5 letters
24 month results
No significant difference between 0.5 mg and 2.0 mg dose
In year 2, PRN treated group had similar visual outcomes with average of 9.9 weeks between injections
0.5 mg dose
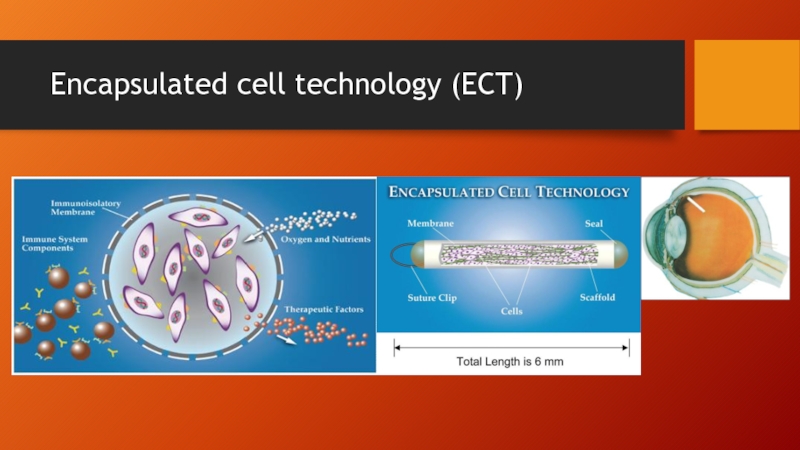
Слайд 37AMD – Where are we going
Encapsulated Cell Technology (Neurotech)
Genetically engineered RPE
Encapsulated non-biodegradable delivery device
Initial device produced ciliary neurotrophic factor (CNTF) for retinitis pigmentosa and dry AMD
Adaptable to produce all classes of biotherapeutics (anti-VEGF, PDGF, etc.)
Слайд 39AMD – Where are we going
Wet AMD/ECT
VEGF receptor decoy (NT-503) –
Phase 1
Dose escalation studies
Double implant vs single
2 line gain with double implant at 10 months
150 micron decrease in central subfield on OCT with double implant vs 50 micron with single
Phase 2 study with new generation implant with 2-3x release rate
Future implants could combine more than 1 therapy (e.g. anti-VEGF + anti-PDGF)
Слайд 40AMD – Where are we going
Nanostructured Tethadur (pSivida)
Implantable silicone porous silicone
Diameter of pores “tuned” to affect sustained release
“Tube of tennis balls”
Faster release with ping pong balls
Slower release with tennis balls
Drug (e.g. Avastin, Eylea, etc) withdrawn from vial and mixed with Tethadur particles
Animal studies – sustained release of bevacizumab for 6 months
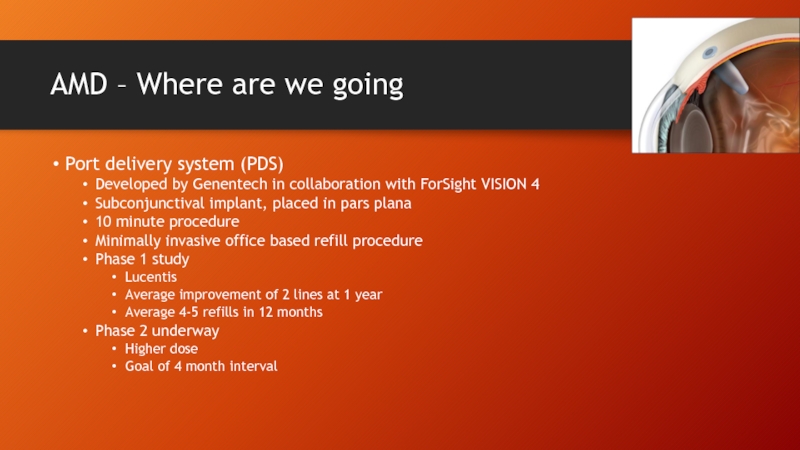
Слайд 42AMD – Where are we going
Port delivery system (PDS)
Developed by Genentech
Subconjunctival implant, placed in pars plana
10 minute procedure
Minimally invasive office based refill procedure
Phase 1 study
Lucentis
Average improvement of 2 lines at 1 year
Average 4-5 refills in 12 months
Phase 2 underway
Higher dose
Goal of 4 month interval
Слайд 43AMD – Where are we going
MicroPump (Replenish)
Battery
Drug reservoir chamber
Refill port accessible
Intraocular canula releases microdose into vitreous cavity
Phase 1 study
Слайд 44AMD – Where are we going
Beyond VEGF
Anti-Platelet derived growth factor (anti-PDGF)
Gene
Topical therapy
Слайд 45Anti-platelet derived growth factor therapy
The problem with anti-VEGF monotherapy
Withdrawal or undertreatment
Anti-VEGF treatment decreases vascular permeability…
BUT does not cause regression of the NV complex
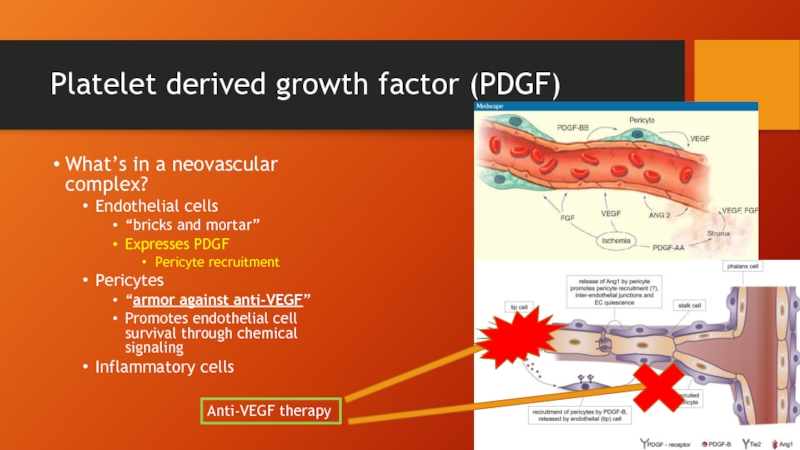
Слайд 47Platelet derived growth factor (PDGF)
What’s in a neovascular complex?
Endothelial cells
“bricks and
Expresses PDGF
Pericyte recruitment
Pericytes
“armor against anti-VEGF”
Promotes endothelial cell survival through chemical signaling
Inflammatory cells
Anti-VEGF therapy
Слайд 51Fovista (Ophthotech)
Pericyte
Anti-VEGF
PDGF
Fovista
Fovista bound to PDGF
Fovista + Anti-VEGF combination therapy
Pericyte coverage protects
Fovista is injected
Fovista binds PDGF leading to stripping away of pericytes
Regression of NV complex!
Ophthotech.com
Слайд 52Fovista (Ophthotech)
Phase 2 study
24 week endpoint
Comparison of Fovista/Lucentis vs Lucentis monotherapy
+10.6
+6.5 for Lucentis monotherapy
Development of subretinal fibrosis
10% in combination group
51% in Lucentis monotherapy group
Phase 3 study underway
Слайд 53Anti-PDGF
Also being developed by:
Neurotech using Encapsulated Cell Technology (ECT) implants
MedImmune as
REGN2176-3 (Regeneron) combination with aflibercept (Eylea) in Phase 1 studies
DE-120 (Santen) anti-VEGF/anti-PDGF in Phase 1 studies
Слайд 55Gene therapy
Therapy in which genetic material is introduced into cells to
Compensate for structurally abnormal or missing genes
Also can be used to induce target host cells to secrete a naturally occurring or engineered therapeutic protein (as alternative to repeated injections)
Слайд 56Gene therapy
Desirable features of viral vectors
Able to introduce and transfer target
No activation of immune response
Vector incapable of causing disease
Much of the vector’s genetic material can be replaced by the therapeutic gene
Adeno-associated virus (AAV)
Слайд 58Gene therapy
Advantages of the eye
It’s small (i.e. low volume, large effect)
Immunologically
Low systemic side effects
Direct delivery of therapy
Слайд 59Ongoing retinal gene therapy trials
Wet AMD
Leber’s congenital amaurosis
Stargardt’s disease
Choroidemia
Usher syndrome
Retinitis pigmentosa
Слайд 60AAV2-sFlt01
Intravitreal Therapy (Genzyme/Sanofi)
Induces expression of a modified VEGF receptor 1
Preclinical primate models
Binds VEGF with high affinity
Expresses VEGFR1 for at least 18 months after 1 injection
Слайд 61AAV2-sFlt01
Subretinal therapy (Avalanche Biotech)
Phase 1 study (6 pts) treated with low/high
2 initial monthly Lucentis injections
Day 380
Слайд 62AAV2-sFlt01
http://permalink.fliqz.com/aspx/permalink.aspx?at=2315379b3136443fa353b26aaf11d582&a=375d1defb6aa477988c6708adf47c1e7
Слайд 63Stem cell transplantation
hESC (human embryonal stem cell)
Pluripotent (any germ layer)
Derived from
Results in destruction of embryo
iPSC (induced pluripotent stem cell)
Derived from adult cells
More tumorigenic than ESC (teratoma formation)
Amniotic and cord blood stem cells
Multipotent
Слайд 65Stem cell transplantation
Ocata Therapeutics (formerly Advanced Cell Technology)
Phase 1/2 studies –
Mean 22 months follow up, 3 dose cohorts (50K, 100K, 150K cells)
Human embroynal stem cell (hESC) derived RPE cells
9 pts c Stargardt’s/9 pts c dry AMD (VA ≤ 20/400 in worse vision cohorts and ≤ 20/100 in better vision cohort)
Wills, Bascom Palmer, UCLA
Слайд 66Stem cell transplantation
Safe, well tolerated (i.e. no rejection by immune system
Stargardt’s: HM to 20/800
dAMD: +7 ETDRS letters
Слайд 67Complement cascade inhibition
Geographic atrophy
Loss of macular RPE
Pathophysiology
Complement cascade hyperactivity (?)
Chronic inflammation/overactivation
Complement factor H and I (CFH and CFI) work together to deactivate the cascade that triggers inflammatory response and cell death
Genetic polymorphisms in CFH, CFH are associated with advanced AMD risk
Слайд 68Complement cascade inhibition
Lampalizumab (Genentech)
Inhibits alternative complement cascade by targeting Complement Factor
MAHALO study, Phase 2 study
Sham vs monthly injection
Primary endopoint – change in GA area
Relationships between genetic polymorphisms with treatment assessed
RESULTS:
20.4% reduction in GA progression in ALL lampalizumab treated pts
44% reduction in GA progression in pts positive for CFI biomarker
57% of DNA tested pts were CFI+
Phase 3 underway
Слайд 71Squalamine
Originally derived from dogfish shark liver, now chemically synthesized
Inhibits both
Initial wet AMD trials
Intravenous infusion
Weekly x 4 weeks, monthly thereafter
Effective gains in VA/maintenance of VA
RAPID plasma clearance – posterior ocular therapeutic concentration requires > 1 IV infusion treatments per week
Слайд 72Squalamine eye drops
Ohr Pharmaceutical
Rapid uptake
Trough levels >> threshold to inhibit angiogenesis
Phase
9 month data
Topical squalamine/Lucentis injection vs sham/Lucentis injection
Initial Lucentis injection and PRN injection thereafter
Слайд 73Retinal vein occlusion (RVO)
Aflibercept (Eylea)
Regeneron
FDA approved for BRVO, October 2014
FDA approved
VIBRANT study, Phase 3
Слайд 74Retinal vein occlusion (RVO)
Similar results for the other anti-VEGFs
Lucentis 0.5 mg
Avastin
Слайд 76Combination treatments
Intravitreal bevacizumab (Avastin)/dexamethasone (Ozurdex)
Maturi, et al. Clin Ophthalmol. 2014; 8:
6 month study
Initial Avastin followed by Ozurdex vs sham
PRN Avastin injection q month
Слайд 78Conclusions
Most patients with wet AMD, DME, RVO can now benefit from
Many patients still fall through the cracks
Poorly responsive
Undertreated
Overall burden of treatment
Knowledge is power
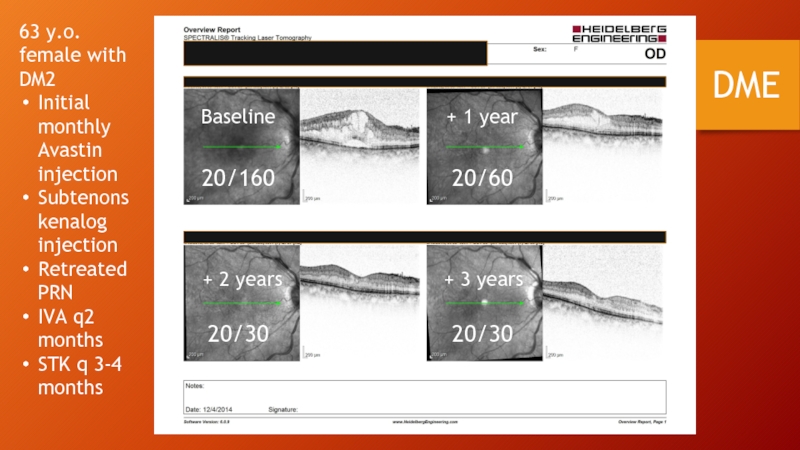
Слайд 79DME
20/160
20/60
20/30
20/30
Baseline
+ 1 year
+ 2 years
+ 3 years
63 y.o. female with DM2
Initial
Subtenons kenalog injection
RetreatedPRN
IVA q2 months
STK q 3-4 months
Слайд 80
67 y.o. male with DM2
Initial monthly Avastin injection
Subtenons kenalog injection
Retreated PRN
IVA
STK q 3-4 months
Baseline
+ 1 year
+ 2 years
20/200
20/40
20/30
DME
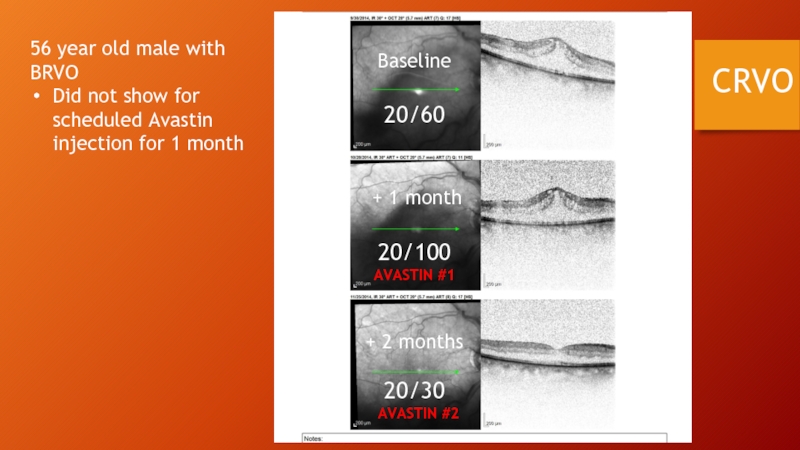
Слайд 81CRVO
56 year old male with BRVO
Did not show for scheduled Avastin
20/60
20/100
20/30
AVASTIN #1
AVASTIN #2
Baseline
+ 1 month
+ 2 months
Слайд 82CRVO
46 y.o. male with CRVO
Initial observation no edema
Baseline
20/40
2 months
4+ months
20/80
AVASTIN #1
20/60
AVASTIN
AVASTIN #3
5+ months
AVASTIN #4
20/30
20/30
20/40
AVASTIN #5
6+ months
7+ months