- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Alkaline earth metals презентация
Содержание
- 1. Alkaline earth metals
- 2. General Properties of 2A *They
- 3. OCCURRENCE Since the group 2A elements are
- 5. Reactions 1) All alkaline earth metals, except
- 6. 2) Ca, Sr and Ba react with
- 7. 3. They form oxides as a
- 8. 4. All alkaline earth metals give
- 9. 5. The reactions of the group 2A
- 10. COMPOUNDS
Слайд 1II – A
GROUP ELEMENTS
Be:Berillium
Mg:Magnesium
Ca:Calcium
Ba:Barium
Sr:Strontim
Ra:Radium
ALKALINE EARTH METALS
Слайд 2 General Properties of 2A
*They give up electrons easily.
*They
have +2 charge
*They are not found free in nature.
*They are malleable.
*They conduct electricity well.
*
Radium is radioactive element
Слайд 3OCCURRENCE
Since the group 2A elements are relatively active metals, they occur
in compounds in nature.
Magnesium, Mg
The principal useful ores o f magnesium are dolomite (CaCO3 · MgCO3 a double salt), carnallite, (KCl · MgCl2 · 6H2O) and epsom salt (MgSO4 · 7H2O) which is found in mineral water.
Magnesium, Mg
The principal useful ores o f magnesium are dolomite (CaCO3 · MgCO3 a double salt), carnallite, (KCl · MgCl2 · 6H2O) and epsom salt (MgSO4 · 7H2O) which is found in mineral water.
Слайд 4
Calcium, Ca
Calcium compounds are widely distributed in nature, occurring as limestone or marble
(CaCO3), gypsum (CaSO4 · 2H2O) and fluorite (CaF2). Salts of sulfate, silicate and phosphate are also found in the earth‘s crust.
Calcium compounds are widely distributed in nature, occurring as limestone or marble
(CaCO3), gypsum (CaSO4 · 2H2O) and fluorite (CaF2). Salts of sulfate, silicate and phosphate are also found in the earth‘s crust.
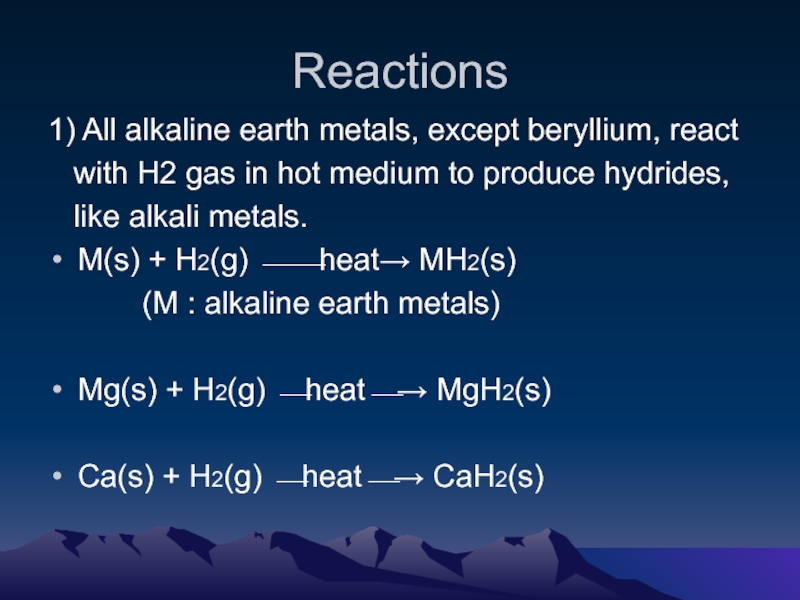
Слайд 5Reactions
1) All alkaline earth metals, except beryllium, react
with H2
gas in hot medium to produce hydrides,
like alkali metals.
M(s) + H2(g) ⎯⎯heat→ MH2(s)
(M : alkaline earth metals)
Mg(s) + H2(g) ⎯heat⎯→ MgH2(s)
Ca(s) + H2(g) ⎯heat⎯→ CaH2(s)
like alkali metals.
M(s) + H2(g) ⎯⎯heat→ MH2(s)
(M : alkaline earth metals)
Mg(s) + H2(g) ⎯heat⎯→ MgH2(s)
Ca(s) + H2(g) ⎯heat⎯→ CaH2(s)
Слайд 62) Ca, Sr and Ba react with water, like alkali metals,
at room temperature to produce metal hydroxides and hydrogen gas.
Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g)
Magnesium metal reacts slowly with boiling water.
The reaction of beryllium with water is very difficult.
Mg(s) + 2H2O(l) ⎯→ Mg(OH)2(s) + H2(g)
Be(s) + 2H2O(l) ⎯→ Be(OH)2(s) + H2(g)
Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g)
Magnesium metal reacts slowly with boiling water.
The reaction of beryllium with water is very difficult.
Mg(s) + 2H2O(l) ⎯→ Mg(OH)2(s) + H2(g)
Be(s) + 2H2O(l) ⎯→ Be(OH)2(s) + H2(g)
Слайд 7
3. They form oxides as a result of their reactions with
oxygen, in MO formula
2M(s) + O2(g) ⎯→ 2MO(s)
2Mg(s) + O2(g) ⎯→ 2MgO(s)
2M(s) + O2(g) ⎯→ 2MO(s)
2Mg(s) + O2(g) ⎯→ 2MgO(s)
Слайд 8
4. All alkaline earth metals give direct reactions with halogens to
produce metal halides.
M(s) + X2(g) ⎯→ MX2(s)
Ca(s) + Cl2(g) ⎯→ CaCl2(s)
Mg(s) + Cl2(g) ⎯→ MgCl2(s)
M(s) + X2(g) ⎯→ MX2(s)
Ca(s) + Cl2(g) ⎯→ CaCl2(s)
Mg(s) + Cl2(g) ⎯→ MgCl2(s)
Слайд 95. The reactions of the group 2A elements with acids like
HCl and H2SO4, produce salts and H2 gas.
Ca(s) + 2HCl(aq) ⎯→ CaCl2(s) + H2(g)
While magnesium reacts with dilute H2SO4 by
giving H2 gas, it reacts with hot and concentrated H2SO4 by producing SO2 gas.
Mg(s) + H2SO4(dil.) → MgSO4(s) + H2(g)
Mg(s) + 2H2SO4(aq)(conc.) → MgSO4(aq) + . SO2(g)+2H20(l)
Ca(s) + 2HCl(aq) ⎯→ CaCl2(s) + H2(g)
While magnesium reacts with dilute H2SO4 by
giving H2 gas, it reacts with hot and concentrated H2SO4 by producing SO2 gas.
Mg(s) + H2SO4(dil.) → MgSO4(s) + H2(g)
Mg(s) + 2H2SO4(aq)(conc.) → MgSO4(aq) + . SO2(g)+2H20(l)