- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
laboratory techniques презентация
Содержание
- 1. laboratory techniques
- 2. CENTRIFUGATION LABORATORY TECHNIQUES
- 3. CENTRIFUGATION DEFINITION ∞ Centrifugation is a process which
- 4. CENTRIFUGATION APPARATUS USED ∞ A centrifuge is a piece
- 5. CHROMATOGRAPHY LABORATORY TECHNIQUES
- 6. CHROMATOGRAPHY DEFINITION ∞ Chromatography is the collective term
- 7. TYPES OF CHROMATOGRAPHY
- 8. CHROMATOGRAPHY: TYPES PAPER CHROMATOGRAPHY
- 9. CHROMATOGRAPHY: TYPES COLUMN CHROMATOGRAPHY
- 10. CHROMATOGRAPHY: TYPES GAS CHROMATOGRAPHY ∞ is a largely
- 11. CHROMATOGRAPHY: TYPES GAS CHROMATOGRAPHY ∞ In this
- 12. CHROMATOGRAPHY TOOLS AND APPARATUS USED The column
- 13. CHROMATOGRAPHY THE MOBILE AND STATIONARY PHASES The
- 14. CHROMATOGRAPHY HOW DOES IT WORK? Think of
- 15. CHROMATOGRAPHY HOW DOES IT WORK? For chromatography
- 16. GEL ELECTROPHORESIS LABORATORY TECHNIQUES
- 17. GEL ELECTROPHORESIS DEFINITION ∞ Gel electrophoresis is
- 18. GEL ELECTROPHORESIS PHYSICAL BASIS In simple terms, electrophoresis is
- 19. GEL ELECTROPHORESIS HISTORY • 1930s – first reports
- 20. TYPES OF GEL
- 21. GEL ELECTROPHORESIS: TYPES OF GEL AGAROSE Agarose
- 22. GEL ELECTROPHORESIS: TYPES OF GEL POLYACRYLAMIDE
- 23. GEL ELECTROPHORESIS: TYPES OF GEL STARCH Partially hydrolysed potato
- 24. GEL ELECTROPHORESIS GEL CONDITIONS ∞ Denaturing gels are
- 25. GEL ELECTROPHORESIS PROCESS Buffers in gel electrophoresis
Слайд 3CENTRIFUGATION
DEFINITION
∞ Centrifugation is a process which involves the application of the centripetal force for
the sedimentation of heterogeneous mixtures with a centrifuge, and is used in industrial and laboratory settings. This process is used to separate two immiscible substances. More-dense components of the mixture migrate away from the axis of the centrifuge, while less-dense components of the mixture migrate towards the axis.
Слайд 4CENTRIFUGATION
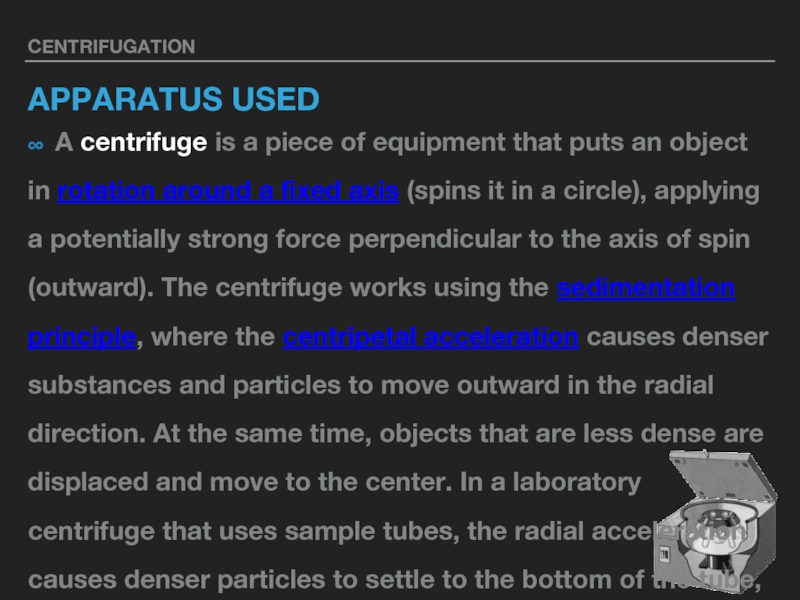
APPARATUS USED
∞ A centrifuge is a piece of equipment that puts an object
in rotation around a fixed axis (spins it in a circle), applying a potentially strong force perpendicular to the axis of spin (outward). The centrifuge works using the sedimentation principle, where the centripetal acceleration causes denser substances and particles to move outward in the radial direction. At the same time, objects that are less dense are displaced and move to the center. In a laboratory centrifuge that uses sample tubes, the radial acceleration causes denser particles to settle to the bottom of the tube, while low-density substances rise to the top.
Слайд 6CHROMATOGRAPHY
DEFINITION
∞ Chromatography is the collective term for a set of laboratory techniques
for the separation of mixtures.
∞ Chromatography may be preparative or analytical.
∞ The purpose of preparative chromatography is to separate the components of a mixture for more advanced use (and is thus a form of purification).
∞ one of the most useful analytical techniques chemists have at their disposal, helpful in everything from identifying biological materials to finding clues at crime scenes.
∞The moving substance is called the mobile phase and the substance that stays put is the stationary phase. As the mobile phase moves, it separates out into its components on the stationary phase.
∞ Chromatography may be preparative or analytical.
∞ The purpose of preparative chromatography is to separate the components of a mixture for more advanced use (and is thus a form of purification).
∞ one of the most useful analytical techniques chemists have at their disposal, helpful in everything from identifying biological materials to finding clues at crime scenes.
∞The moving substance is called the mobile phase and the substance that stays put is the stationary phase. As the mobile phase moves, it separates out into its components on the stationary phase.
Слайд 8CHROMATOGRAPHY: TYPES
PAPER CHROMATOGRAPHY
∞ This is the "spot of ink on paper" experiment you often do in school (also the effect we described at the start when you get your papers wet). Typically you put a spot of ink near one edge of some filter paper and then hang the paper vertically with its lower edge (nearest the spot) dipped in a solvent such as alcohol or water. Capillary action makes the solvent travel up the paper, where it meets and dissolves the ink. The dissolved ink (the mobile phase) slowly travels up the paper (the stationary phase and separates out into different components. Sometimes these are colored; sometimes you have to color them by adding other substances (called developers or developing fluids) that help you with identification.
Слайд 9CHROMATOGRAPHY: TYPES
COLUMN CHROMATOGRAPHY
∞ Instead of paper, the stationary phase is a vertical glass jar (the column) packed with a highly adsorbent solid, such as crystals of silica or silica gel, or a solid coated with a liquid. The mobile phase is pumped at high pressure through the column and splits into its components, which are then removed and analyzed. In liquid-column chromatography, the mixture being studied is placed at one end of the column and an extra added substance called an eluant is poured in to help it travel through. Thin-film chromatography is a variation of this technique in which the "column" is actually a film of glass, plastic, or metal coated with a very thin layer of adsorbent material
Слайд 10CHROMATOGRAPHY: TYPES
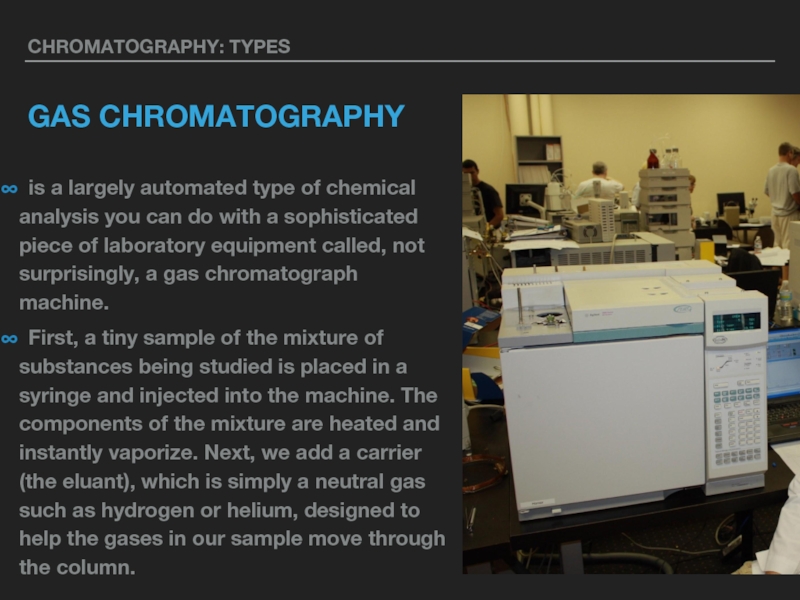
GAS CHROMATOGRAPHY
∞ is a largely automated type of chemical analysis you
can do with a sophisticated piece of laboratory equipment called, not surprisingly, a gas chromatograph machine.
∞ First, a tiny sample of the mixture of substances being studied is placed in a syringe and injected into the machine. The components of the mixture are heated and instantly vaporize. Next, we add a carrier (the eluant), which is simply a neutral gas such as hydrogen or helium, designed to help the gases in our sample move through the column.
∞ First, a tiny sample of the mixture of substances being studied is placed in a syringe and injected into the machine. The components of the mixture are heated and instantly vaporize. Next, we add a carrier (the eluant), which is simply a neutral gas such as hydrogen or helium, designed to help the gases in our sample move through the column.
Слайд 11CHROMATOGRAPHY: TYPES
GAS CHROMATOGRAPHY
∞ In this case, the column is a thin
glass or metal tube usually filled with a liquid that has a high boiling point (or sometimes a gel or an adsorbent solid). As the mixture travels through the column, it's adsorbed and separates out into its components. Each component emerges in turn from the end of the column and moves past an electronic detector (sometimes a mass spectrometer), which identifies it and prints a peak on a chart. The final chart has a series of peaks that correspond to all the substances in the mixture. Gas chromatography is sometimes called vapor-phase chromatography (VPC) or gas-liquid partition chromatography (GLPC).
Слайд 12CHROMATOGRAPHY
TOOLS AND APPARATUS USED
The column is where the actual separation takes
place. It is usually a glass or metal of with sufficient strength to handle pressure.
A packed bed column in compromised of a stationary phase which is granular form and packed into the column as homogenous bed. The stationary phase complete fills the column.
An open tubular column’s stationary phase is a thin film or layer on the column wall.
A packed bed column in compromised of a stationary phase which is granular form and packed into the column as homogenous bed. The stationary phase complete fills the column.
An open tubular column’s stationary phase is a thin film or layer on the column wall.
Слайд 13CHROMATOGRAPHY
THE MOBILE AND STATIONARY PHASES
The mobile phase is comprised of a
solvent into which the sample is injected. The solvent and sample flow through the column together; thus the mobile phase is often referred to as the "carrier fluid." The stationary phase is the material in the column for which the components to be separated have varying affinities. The materials which comprise the mobile and stationary phases vary depending on the general type of chromatographic process being performed.
Слайд 14CHROMATOGRAPHY
HOW DOES IT WORK?
Think of chromatography as a race and you'll
find it's much simpler than it sounds. Waiting on the starting line, you've got a mixture of chemicals in some unidentified liquid or gas, just like a load of runners all mixed up and bunched together. When a race starts, runners soon spread out because they have different abilities. In exactly the same way, chemicals in something like a moving liquid mixture spread out because they travel at different speeds over a stationary solid. The key thing to remember is that chromatography is a surface effect.
Слайд 15CHROMATOGRAPHY
HOW DOES IT WORK?
For chromatography to work effectively, we obviously need
the components of the mobile phase to separate out as much as possible as they move past the stationary phase. That's why the stationary phase is often something with a large surface area, such as a sheet of filter paper, a solid made of finely divided particles, a liquid deposited on the surface of a solid, or some other highly adsorbent material.
The mixture is dissolved in a fluid called the mobile phase, which carries it through a structure holding another material called the stationary phase. Different rates of migration cause the various constituents of the mixture to travel at different speeds, causing them to separate.
The mixture is dissolved in a fluid called the mobile phase, which carries it through a structure holding another material called the stationary phase. Different rates of migration cause the various constituents of the mixture to travel at different speeds, causing them to separate.
Слайд 17GEL ELECTROPHORESIS
DEFINITION
∞ Gel electrophoresis is a method for separation and analysis
of macromolecules (DNA, RNA and proteins) and their fragments, based on their size and charge. It is used in clinical chemistry to separate proteins by charge and/or size (IEF agarose, essentially size independent) and in biochemistry and molecular biology to separate a mixed population of DNA and RNA fragments by length, to estimate the size of DNA and RNA fragments or to separate proteins by charge.
∞ Nucleic acid molecules are separated by applying an electric field to move the negatively charged molecules through a matrix of agarose or other substances. Shorter molecules move faster and migrate farther than longer ones because shorter molecules migrate more easily through the pores of the gel. This phenomenon is called sieving.
∞ Proteins are separated by charge in agarose because the pores of the gel are too large to sieve proteins.
∞ Nucleic acid molecules are separated by applying an electric field to move the negatively charged molecules through a matrix of agarose or other substances. Shorter molecules move faster and migrate farther than longer ones because shorter molecules migrate more easily through the pores of the gel. This phenomenon is called sieving.
∞ Proteins are separated by charge in agarose because the pores of the gel are too large to sieve proteins.
Слайд 18GEL ELECTROPHORESIS
PHYSICAL BASIS
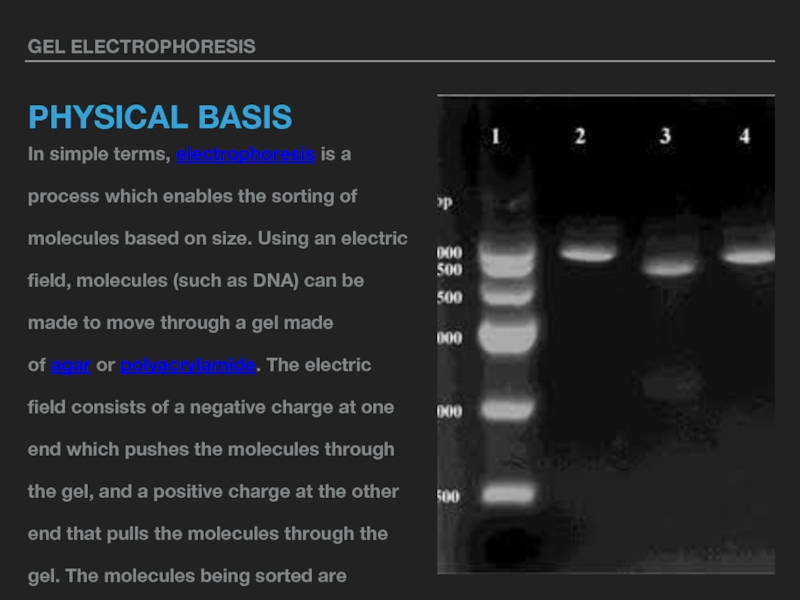
In simple terms, electrophoresis is a process which enables the sorting
of molecules based on size. Using an electric field, molecules (such as DNA) can be made to move through a gel made of agar or polyacrylamide. The electric field consists of a negative charge at one end which pushes the molecules through the gel, and a positive charge at the other end that pulls the molecules through the gel. The molecules being sorted are dispensed into a well in the gel material. The gel is placed in an electrophoresis chamber, which is then connected to a power source. When the electric current is applied, the larger molecules move more slowly through the gel while the smaller molecules move faster. The different sized molecules form distinct bands on the gel.
Слайд 19GEL ELECTROPHORESIS
HISTORY
• 1930s – first reports of the use of sucrose for
gel electrophoresis
• 1955 – introduction of starch gels, mediocre separation
• 1959 – introduction of acrylamide gels; disc electrophoresis (Ornstein and Davis); accurate control of parameters such as pore size and stability; and (Raymond and Weintraub)
• 1966 – agar gels
• 1969 – introduction of denaturing agents especially SDS separation of protein subunit (Weber and Osborn)
• 1970 – Laemmli separated 28 components of T4 phage using a stacking gel and SDS
• 1972 – agarose gels with ethidium bromide stain
• 1975 – 2-dimensional gels (O’Farrell); isoelectric focusing then SDS gel electrophoresis
• 1977 – sequencing gels
• 1983 – pulsed field gel electrophoresis enables separation of large DNA molecules
• 1983 – introduction of capillary electrophoresis
• 2004 – standardized time of polymerization of acrylamide gels enables clean and predictable separation of native proteins
• 1955 – introduction of starch gels, mediocre separation
• 1959 – introduction of acrylamide gels; disc electrophoresis (Ornstein and Davis); accurate control of parameters such as pore size and stability; and (Raymond and Weintraub)
• 1966 – agar gels
• 1969 – introduction of denaturing agents especially SDS separation of protein subunit (Weber and Osborn)
• 1970 – Laemmli separated 28 components of T4 phage using a stacking gel and SDS
• 1972 – agarose gels with ethidium bromide stain
• 1975 – 2-dimensional gels (O’Farrell); isoelectric focusing then SDS gel electrophoresis
• 1977 – sequencing gels
• 1983 – pulsed field gel electrophoresis enables separation of large DNA molecules
• 1983 – introduction of capillary electrophoresis
• 2004 – standardized time of polymerization of acrylamide gels enables clean and predictable separation of native proteins
Слайд 21GEL ELECTROPHORESIS: TYPES OF GEL
AGAROSE
Agarose gels are made from the natural polysaccharide polymers extracted
from seaweed. Agarose gels are easily cast and handled compared to other matrices, because the gel setting is a physical rather than chemical change. Samples are also easily recovered. After the experiment is finished, the resulting gel can be stored in a plastic bag in a refrigerator.
Agarose gels do not have a uniform pore size, but are optimal for electrophoresis of proteins that are larger than 200 kDa. Agarose gel electrophoresis can also be used for the separation of DNA fragments ranging from 50 base pair to several megabases (millions of bases), the largest of which require specialized apparatus.
Agarose gels do not have a uniform pore size, but are optimal for electrophoresis of proteins that are larger than 200 kDa. Agarose gel electrophoresis can also be used for the separation of DNA fragments ranging from 50 base pair to several megabases (millions of bases), the largest of which require specialized apparatus.
Слайд 22GEL ELECTROPHORESIS: TYPES OF GEL
POLYACRYLAMIDE
Polyacrylamide gel electrophoresis (PAGE) is used
for separating proteins ranging in size from 5 to 2,000 kDa due to the uniform pore size provided by the polyacrylamide gel. Pore size is controlled by modulating the concentrations of acrylamide and bis-acrylamide powder used in creating a gel. Care must be used when creating this type of gel, as acrylamide is a potent neurotoxin in its liquid and powdered forms.
Traditional DNA sequencing techniques such as Maxam-Gilbert or Sanger methods used polyacrylamide gels to separate DNA fragments differing by a single base-pair in length so the sequence could be read. Most modern DNA separation methods now use agarose gels, except for particularly small DNA fragments. It is currently most often used in the field of immunology and protein analysis, often used to separate different proteins or isoforms of the same protein into separate bands
Traditional DNA sequencing techniques such as Maxam-Gilbert or Sanger methods used polyacrylamide gels to separate DNA fragments differing by a single base-pair in length so the sequence could be read. Most modern DNA separation methods now use agarose gels, except for particularly small DNA fragments. It is currently most often used in the field of immunology and protein analysis, often used to separate different proteins or isoforms of the same protein into separate bands
Слайд 23GEL ELECTROPHORESIS: TYPES OF GEL
STARCH
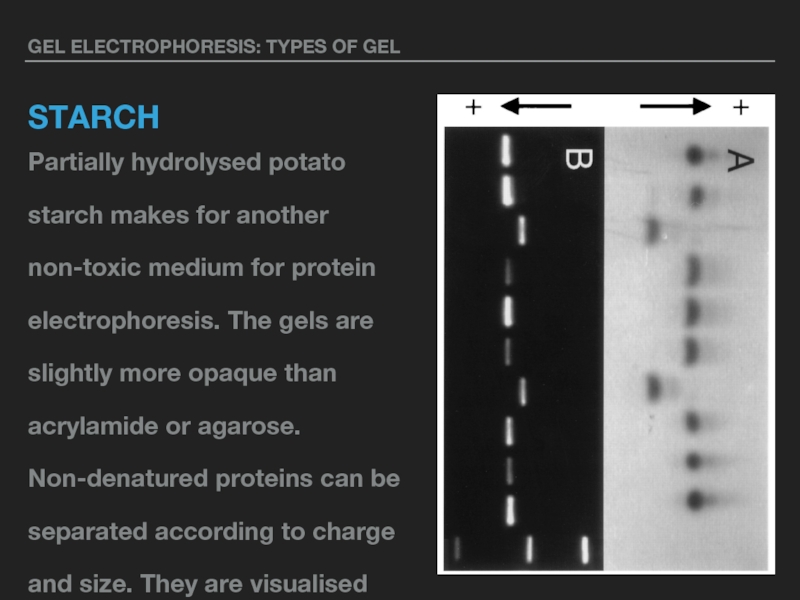
Partially hydrolysed potato starch makes for another non-toxic medium
for protein electrophoresis. The gels are slightly more opaque than acrylamide or agarose. Non-denatured proteins can be separated according to charge and size. They are visualised using Napthal Black or Amido Black staining. Typical starch gel concentrations are 5% to 10%.
Слайд 24GEL ELECTROPHORESIS
GEL CONDITIONS
∞ Denaturing gels are run under conditions that disrupt the
natural structure of the analyte, causing it to unfold into a linear chain. Thus, the mobility of each macromolecule depends only on its linear length and its mass-to-charge ratio. Thus, the secondary, tertiary, and quaternary levels of biomolecular structure are disrupted, leaving only the primary structure to be analyzed.
∞ Native gels are run in non-denaturing conditions, so that the analyte's natural structure is maintained. This allows the physical size of the folded or assembled complex to affect the mobility, allowing for analysis of all four levels of the biomolecular structure. For biological samples, detergents are used only to the extent that they are necessary to lyse lipid membranes in the cell. Complexes remain—for the most part—associated and folded as they would be in the cell. One downside, however, is that complexes may not separate cleanly or predictably, as it is difficult to predict how the molecule's shape and size will affect its mobility.
∞ Native gels are run in non-denaturing conditions, so that the analyte's natural structure is maintained. This allows the physical size of the folded or assembled complex to affect the mobility, allowing for analysis of all four levels of the biomolecular structure. For biological samples, detergents are used only to the extent that they are necessary to lyse lipid membranes in the cell. Complexes remain—for the most part—associated and folded as they would be in the cell. One downside, however, is that complexes may not separate cleanly or predictably, as it is difficult to predict how the molecule's shape and size will affect its mobility.
Слайд 25GEL ELECTROPHORESIS
PROCESS
Buffers in gel electrophoresis are used to provide ions that
carry a current and to maintain the pH at a relatively constant value. There are a number of buffers used for electrophoresis. The most common being, for nucleic acids Tris/Acetate/EDTA (TAE), Tris/Borate/EDTA (TBE).
After the electrophoresis is complete, the molecules in the gel can be stained to make them visible. DNA may be visualized using ethidium bromide which, when intercalated into DNA, fluoresce under ultraviolet light, while protein may be visualised using silver stain or Coomassie Brilliant Blue dye.
After separation, an additional separation method may then be used, such as isoelectric focusing or SDS-PAGE. The gel will then be physically cut, and the protein complexes extracted from each portion separately. Each extract may then be analysed, such as by peptide mass fingerprinting or de novo peptide sequencing after in-gel digestion. This can provide a great deal of information about the identities of the proteins in a complex.
After the electrophoresis is complete, the molecules in the gel can be stained to make them visible. DNA may be visualized using ethidium bromide which, when intercalated into DNA, fluoresce under ultraviolet light, while protein may be visualised using silver stain or Coomassie Brilliant Blue dye.
After separation, an additional separation method may then be used, such as isoelectric focusing or SDS-PAGE. The gel will then be physically cut, and the protein complexes extracted from each portion separately. Each extract may then be analysed, such as by peptide mass fingerprinting or de novo peptide sequencing after in-gel digestion. This can provide a great deal of information about the identities of the proteins in a complex.