- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Pancreatic Cancer презентация
Содержание
- 1. Pancreatic Cancer
- 2. Topics Part 1 Epidemiology Pathology Risk
- 3. Cancer statistics CA: A Cancer
- 4. USA statistics The American Cancer Society's
- 5. Overall incidence of pancreatic cancer is
- 6. Incidence in Israel
- 8. EXOCRINE AND ENDOCRINE ORGAN
- 9. Pathology Exocrine tumors Solid Cystic Endocrine tumors
- 10. Solid Epithelial Tumors Adenocarcinomas: 75-80%, white yellow,
- 11. Infiltrate into vascular, lymphatic, perineural spaces.
- 12. GENETICS OF PANCREATIC CANCER
- 14. Nature 467, 1114-1117 (28 October 2010)
- 15. A quantitative analysis of the timing of
- 16. Hidalgo M. N Engl J Med 2010;362:1605-1617 Components of Pancreatic Cancer
- 17. RISK FACTORS Advanced age Smoking diet Chronic
- 18. Age Age is the most significant
- 19. The age-specific incidence rates of pancreatic cancer
- 20. RISK FACTORS Advanced age Smoking diet Chronic
- 21. Smoking Associated with 20-25% of PC
- 22. RISK FACTORS Advanced age Smoking diet Chronic
- 23. Obesity & nutrition High caloric intake &
- 24. Anthropometric Measures, Body Mass Index, and Pancreatic
- 25. Obesity & nutrition High caloric intake &
- 26. Alcohol Intake and Pancreatic Cancer Risk:
- 27. RISK FACTORS Advanced age Smoking diet Chronic
- 28. 14-fold increased risk of PC in
- 29. RISK FACTORS Advanced age Smoking diet Chronic
- 30. Increased risk of PC in type
- 31. RISK FACTORS Advanced age Smoking diet Chronic
- 32. ABO Blood Group and the Risk of
- 33. RISK FACTORS Advanced age Smoking diet Chronic
- 34. Inherited pancreatic cancer An inherited tendency to
- 35. Familial pancreatic cancer Familial pancreatic cancer (FPC)
- 36. Genetic syndromes
- 37. Both BRCA1 (breast cancer gene1) and
- 38. Pancreatic cancer in BRCA1/2 Risk of PC
- 39. BRCA1/2 in pancreatic cancer BRCA2 in
- 40. BRCA1/2 in pancreatic cancer RAMABM HCC BRCA1/2
- 41. Low risk (less than 5-fold) Factors
- 42. Moderate risk (5 to10-fold) Factors Family
- 43. High risk (greater than 10-fold) Factors
- 44. BRCA1/2 in pancreatic cancer RAMABM HCC
- 45. How to screen? Which strategy should
- 46. Clinical course and treatment
- 47. Pancreatic Cancer- diagnosis: Symptoms Symptoms
- 48. Pancreatic Cancer- Diagnosis: imaging and lab
- 49. Staging Tram et al. “Diagnosis, Staging, and
- 52. Pancreatic cancer: stage at diagnosis 10 -
- 53. Pancreatic cancer Survival
- 54. Why are the results so poor
- 55. Treatment of metastatic pancreatic cancer
- 57. Pts = 126 Treatment Schedule Gemcitabine 1000mg/m2/wk 5-Fluorouracil (5FU) 600mg/m2/wk
- 58. Metastatic pancreatic cancer Gemcitabine No confirmed objective
- 59. Beyond single-agent gemcitabine ? Gemcitabine-based combination CT
- 60. 0 0,25 0,5 0,75 1 0 6
- 61. Beyond single-agent gemcitabine ? Gemcitabine-based combination CT
- 63. GEM plus Erlotinib 6.24 months (GEM+ERL) vs.
- 64. GEM plus Erlotinib
- 65. Locally advanced disease (LAD) clinical highlights
- 66. LAD Aims of treatment Improvement of
- 67. Practical guidelines 2013 Rambam Gemcitabine-based chemotherapy for
- 68. The Whipple Resection Specimen (Pancreaticoduodenal resection)
- 69. אלבום תמונות על ידי אר
- 70. Resectable pancreatic cancer Long-term survival after resection
- 71. overall survival among all 1,092 resected pancreatic
- 72. Adjuvant chemoradiotherapy – randomized studies (2) ESPAC-1
- 74. Resectable pancreatic cancer adjuvant therapy chemotherapy only?
- 76. Practical guidelines 2014 Rambam Medical Center Chemoradiation
- 77. Still unclear… Pancreatology. 2012 Mar;12(2):162-9. Epub 2012
- 78. Conclusions: A significant benefit with regard
- 79. Future directions The future is here, now…?
- 80. Personalized medicine patients with the same cancer
- 81. Personalized medicine Gene expression profiling, molecular profiling,
- 82. RRM1 → Gemcitabine RRM1 (Ribonucleotide
- 83. SPARC (Secreted Protein Acidic and Rich
- 84. SPARC
- 85. Evidence for SPARC as a biomarker
- 86. BRCA1/2 m → PARP inhibitors
- 87. immunohistochemistry (IHC) analysis: level of important
- 88. Target Now A comprehensive patient’s tumor analysis
- 89. A Retrospective Investigation to Evaluate the Use
- 90. Druggable targets reported included Molecular Profiling Identifies Actionable Targets (n=20 patients)
- 91. Molecular Profiling Identifies Potential Therapeutic Options in Advanced Pancreatic Cancer (n=20 patients)
- 92. Molecular Profiling Guided Treatment Choices (n=20
- 93. Nab-paclitaxel Capecitabine + Irinotecan Capecitabine Gemcitabine +Oxaliplatin Nab-paclitaxel Capecitabine Capecitabine
- 94. During the progression of metastasis, cancer cells
- 95. Analysis of CTCs Yu et al. (2011) J Cell Biol
- 96. M Murtaza et al. Nature April 7
Слайд 1Pancreatic Cancer- 2017
Valeriya Semenisty
Department of Oncology,
Rambam Medical Center,
Haifa, Israel
Слайд 2Topics
Part 1
Epidemiology
Pathology
Risk factors
Genetics
Part 2
Clinical course
Treatment
Metastatic disease
Locally advanced non-resectable tumor
Resectable tumor
Part
Personalized treatment
Imaging
Слайд 3Cancer statistics
CA: A Cancer Journal for Clinicians
Volume 63, Issue 1, pages
Слайд 4
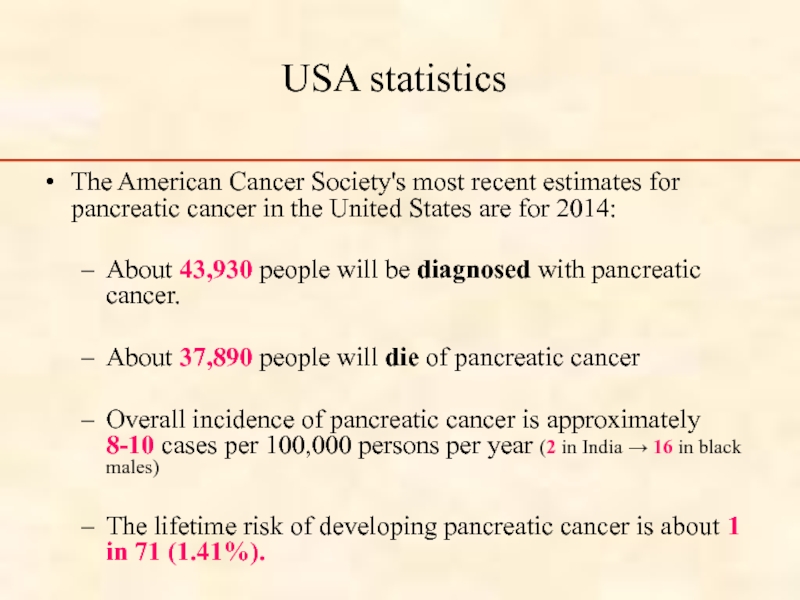
USA statistics
The American Cancer Society's most recent estimates for pancreatic cancer
About 43,930 people will be diagnosed with pancreatic cancer.
About 37,890 people will die of pancreatic cancer
Overall incidence of pancreatic cancer is approximately 8-10 cases per 100,000 persons per year (2 in India → 16 in black males)
The lifetime risk of developing pancreatic cancer is about 1 in 71 (1.41%).
Слайд 5
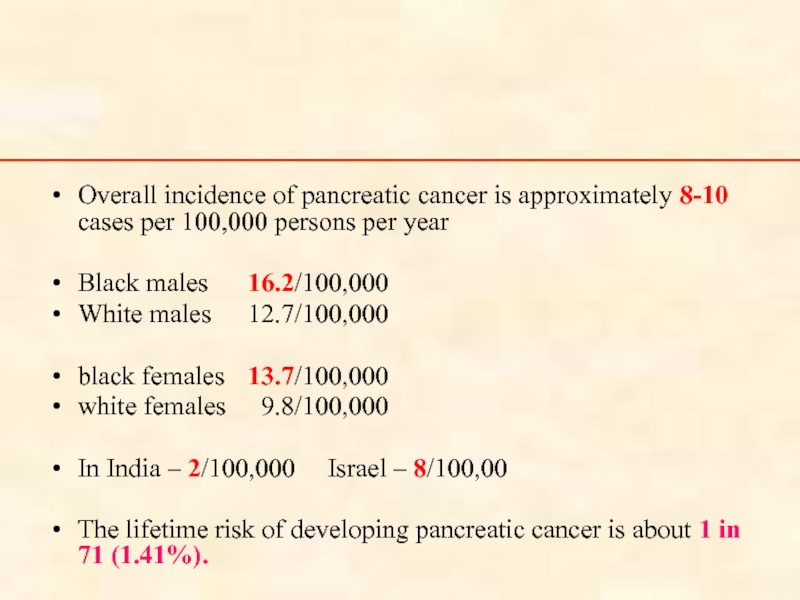
Overall incidence of pancreatic cancer is approximately 8-10 cases per 100,000
Black males 16.2/100,000
White males 12.7/100,000
black females 13.7/100,000
white females 9.8/100,000
In India – 2/100,000 Israel – 8/100,00
The lifetime risk of developing pancreatic cancer is about 1 in 71 (1.41%).
Слайд 10Solid Epithelial Tumors
Adenocarcinomas: 75-80%, white yellow, poorly defined, often obstruct bile
Often associated with a desmoplastic reaction that causes fibrosis and chronic pancreatitis.
Слайд 11
Infiltrate into vascular, lymphatic, perineural spaces.
At resection, most mets to lymph
Mets to liver (80%), peritoneum (60%), lungs and pleura (50-70%), adrenal (25%). Direct invasion of adjacent organs as well.
Others include adenosquamous, acinar cell (1%, better prognosis), giant cell (5%, poorer prognosis), pancreatoblastoma (children 1-15 years, more favorable).
Слайд 14
Nature 467, 1114-1117 (28 October 2010)
Distant metastasis occurs late during the
Shinichi Yachida1Shinichi Yachida1et al7,
Department of Pathology, The Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins Medical Institutions, Baltimore, Maryland 21231, USA
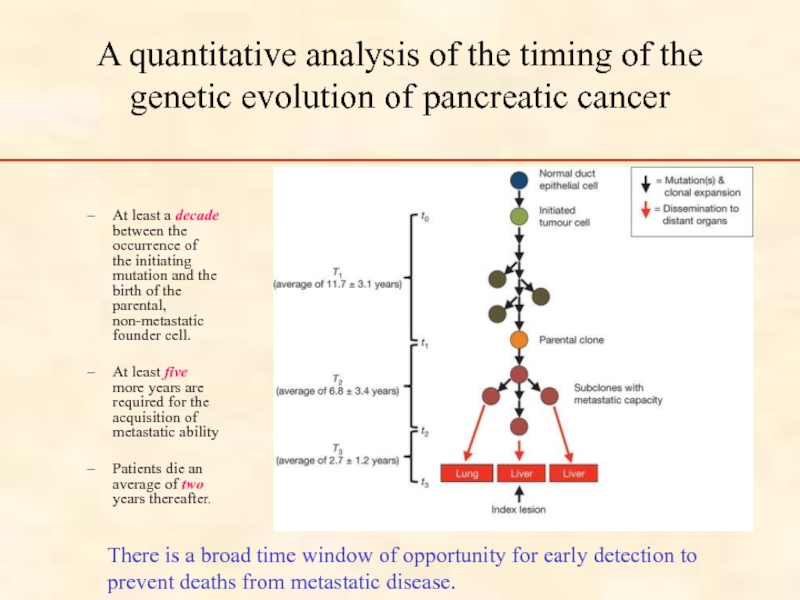
Слайд 15A quantitative analysis of the timing of the genetic evolution of
At least a decade between the occurrence of the initiating mutation and the birth of the parental, non-metastatic founder cell.
At least five more years are required for the acquisition of metastatic ability
Patients die an average of two years thereafter.
There is a broad time window of opportunity for early detection to prevent deaths from metastatic disease.
Слайд 17RISK FACTORS
Advanced age
Smoking
diet
Chronic pancreatitis
Diabetes mellitus
Blood type A, B, AB
Family history
Слайд 18Age
Age is the most significant risk factor for pancreatic cancer.
In the absence of predisposing conditions pancreatic cancer is unusual in persons younger than 45 years. Only 10% of patients are diagnosed when younger than 50 years of age.
After age 50 years, the frequency of pancreatic cancer increases linearly.
The median age at diagnosis is 69 years in whites and 65 years in blacks
Слайд 19The age-specific incidence rates of pancreatic cancer in different racial groups
pancreatic
Слайд 20RISK FACTORS
Advanced age
Smoking
diet
Chronic pancreatitis
Diabetes mellitus
Blood type A, B, AB
Family history
Слайд 21Smoking
Associated with 20-25% of PC cases
People who smoke have 2.7-3.7 -fold
Current smokers with over a 40 pack-year history of smoking may have up to a 5-fold increase risk of the disease.
It takes 5-10 years of discontinued smoking to reduce the increased risk of smoking to approximately that of nonsmokers.
Слайд 22RISK FACTORS
Advanced age
Smoking
diet
Chronic pancreatitis
Diabetes mellitus
Blood type A, B, AB
Family history
Слайд 23Obesity & nutrition
High caloric intake & obesity are risk factors for
Red meat consumption, especially processed, is associated with a higher risk of pancreatic cancer
Слайд 24Anthropometric Measures, Body Mass Index, and Pancreatic Cancer A Pooled Analysis
A positive association between increasing BMI and risk of pancreatic cancer was observed (adjusted OR for the highest vs lowest BMI quartile, 1.33; 95% CI, 1.12-1.58; Ptrend < .001).
Increased waist to hip ratio was associated with increased risk of pancreatic cancer in women (adjusted OR for the highest vs lowest quartile, 1.87; 95% CI, 1.31-2.69; Ptrend = .003) but less so in men.
Слайд 25Obesity & nutrition
High caloric intake & obesity are risk factors for
Red meat consumption, especially processed, is associated with a higher risk of pancreatic cancer
Слайд 26
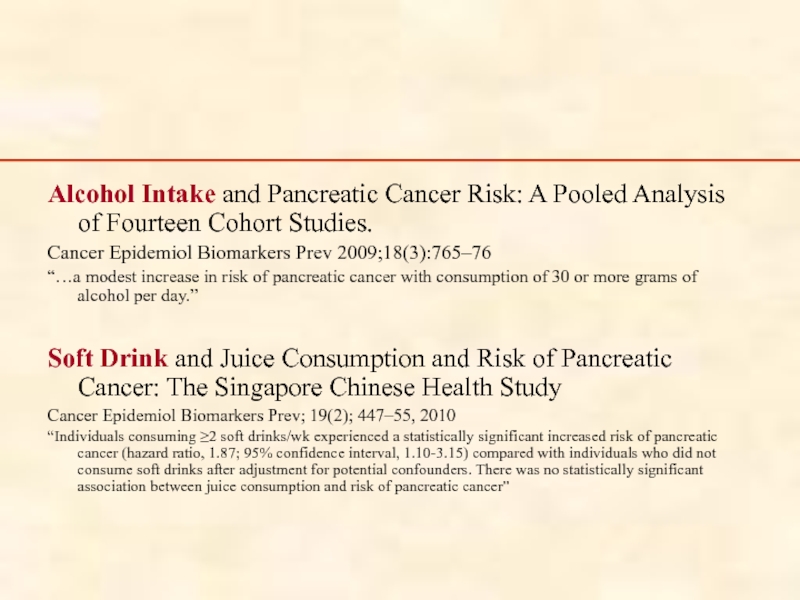
Alcohol Intake and Pancreatic Cancer Risk: A Pooled Analysis of Fourteen
Cancer Epidemiol Biomarkers Prev 2009;18(3):765–76
“…a modest increase in risk of pancreatic cancer with consumption of 30 or more grams of alcohol per day.”
Soft Drink and Juice Consumption and Risk of Pancreatic Cancer: The Singapore Chinese Health Study
Cancer Epidemiol Biomarkers Prev; 19(2); 447–55, 2010
“Individuals consuming ≥2 soft drinks/wk experienced a statistically significant increased risk of pancreatic cancer (hazard ratio, 1.87; 95% confidence interval, 1.10-3.15) compared with individuals who did not consume soft drinks after adjustment for potential confounders. There was no statistically significant association between juice consumption and risk of pancreatic cancer”
Слайд 27RISK FACTORS
Advanced age
Smoking
diet
Chronic pancreatitis
Diabetes mellitus
Blood type A, B, AB
Family history
Слайд 28
14-fold increased risk of PC in chronic pancreatitis patients
Hereditary pancreatitiis →
Слайд 29RISK FACTORS
Advanced age
Smoking
diet
Chronic pancreatitis
Diabetes mellitus
Blood type A, B, AB
Family history
Слайд 30
Increased risk of PC in type II diabetes (RR 2.1-2.6)
Etiologic factor
Manifestation of PC ?
Слайд 31RISK FACTORS
Advanced age
Smoking
diet
Chronic pancreatitis
Diabetes mellitus
Blood type A, B, AB
Family history
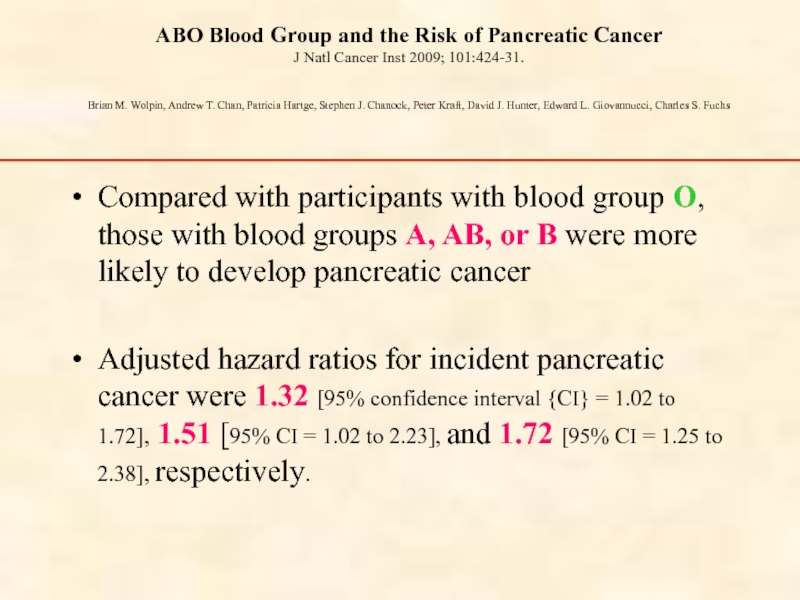
Слайд 32ABO Blood Group and the Risk of Pancreatic Cancer J Natl Cancer
Compared with participants with blood group O, those with blood groups A, AB, or B were more likely to develop pancreatic cancer
Adjusted hazard ratios for incident pancreatic cancer were 1.32 [95% confidence interval {CI} = 1.02 to 1.72], 1.51 [95% CI = 1.02 to 2.23], and 1.72 [95% CI = 1.25 to 2.38], respectively.
Слайд 33RISK FACTORS
Advanced age
Smoking
diet
Chronic pancreatitis
Diabetes mellitus
Blood type A, B, AB
Family history
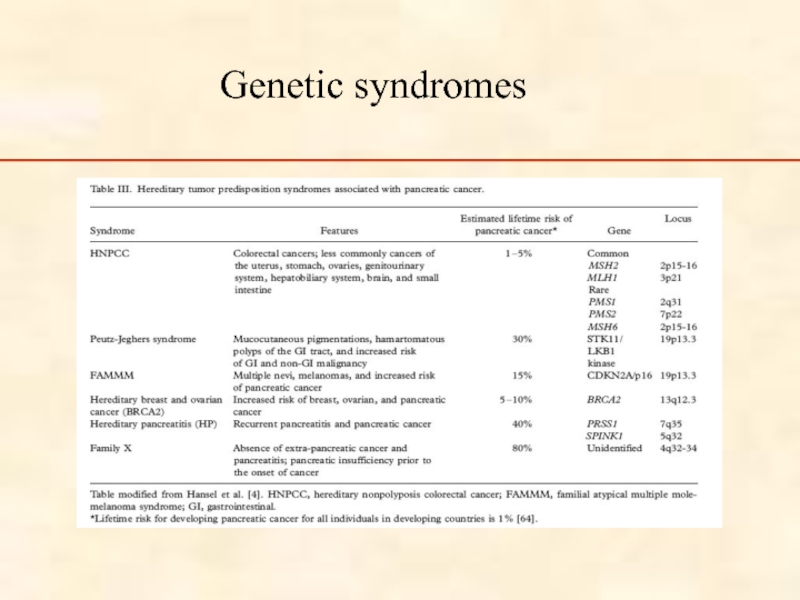
Слайд 34Inherited pancreatic cancer
An inherited tendency to develop this cancer may occur
Minority (< 20%) of inherited pancreatic cancers are associated with known genetic syndromes
Слайд 35Familial pancreatic cancer
Familial pancreatic cancer (FPC) = >2 first degree family
PC in one 1st degree relative: RR= 4.6 (lifetime risk 6%)
PC in 2 1st degree relatives: RR= 6.4-9.0 (8-12%)
In ≥ 3 1st degree relatives RR= 32 (40%)
Слайд 37
Both BRCA1 (breast cancer gene1) and BRCA2 are tumor suppressor genes
Related mainly to breast and ovarian cancers.
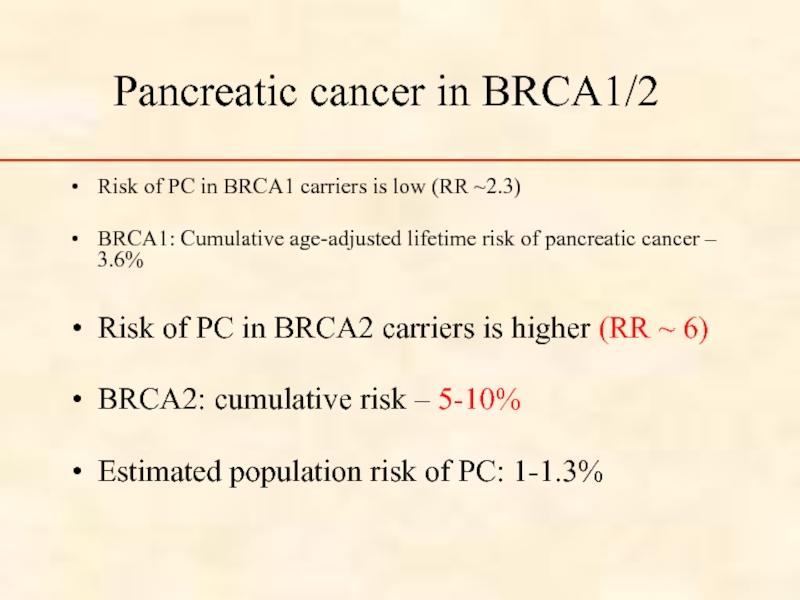
Слайд 38Pancreatic cancer in BRCA1/2
Risk of PC in BRCA1 carriers is low
BRCA1: Cumulative age-adjusted lifetime risk of pancreatic cancer – 3.6%
Risk of PC in BRCA2 carriers is higher (RR ~ 6)
BRCA2: cumulative risk – 5-10%
Estimated population risk of PC: 1-1.3%
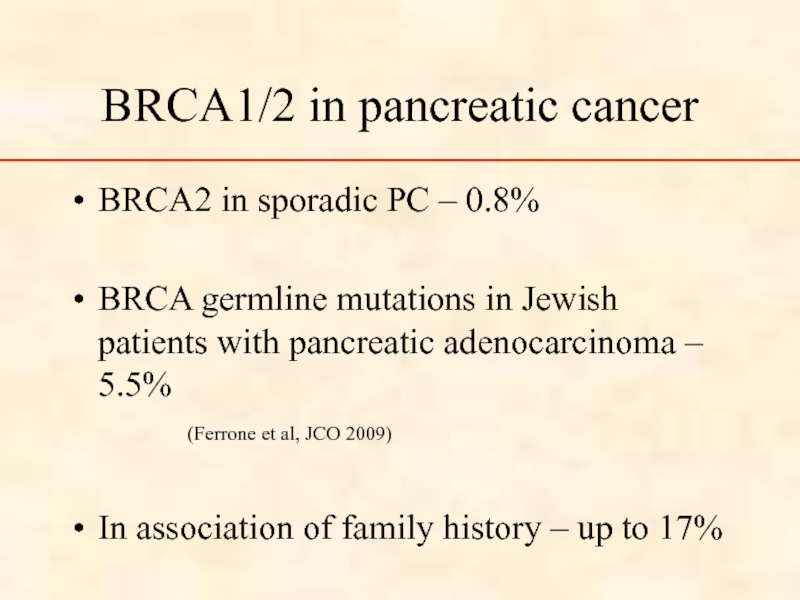
Слайд 39BRCA1/2 in pancreatic cancer
BRCA2 in sporadic PC – 0.8%
BRCA germline
(Ferrone et al, JCO 2009)
In association of family history – up to 17%
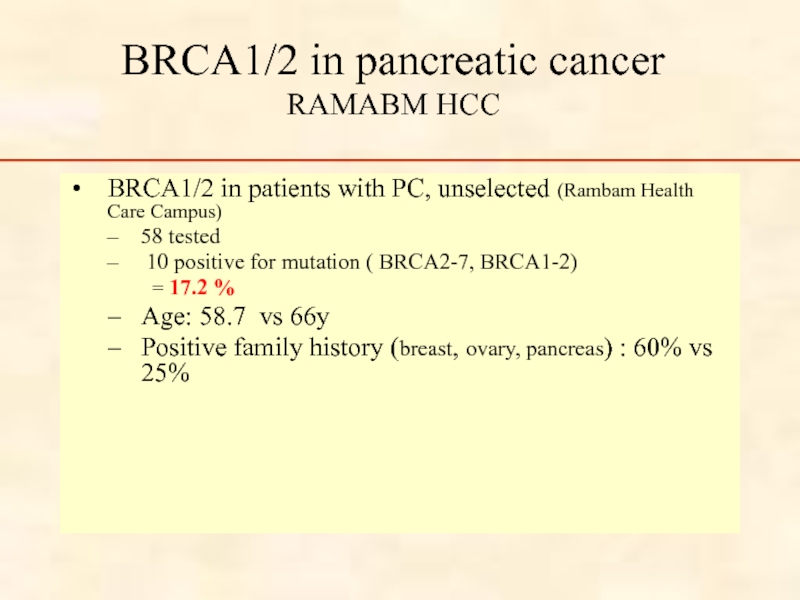
Слайд 40BRCA1/2 in pancreatic cancer
RAMABM HCC
BRCA1/2 in patients with PC, unselected (Rambam
58 tested
10 positive for mutation ( BRCA2-7, BRCA1-2)
= 17.2 %
Age: 58.7 vs 66y
Positive family history (breast, ovary, pancreas) : 60% vs 25%
Слайд 41Low risk
(less than 5-fold)
Factors
Race/sex:
male
black
Ashkenazi Jewish descent
Exposures:
obesity
smoking
diabetes
Helicobacter pylori infection
Family history:
cancer history in a first-degree relative
history of pancreatic cancer in one first-degree relative
Inherited conditions:
hereditary non-polyposis colorectal cancer
familial adenomatous polyposis
BRCA1 mutation carrier
Brand RE et al, Gut 2007
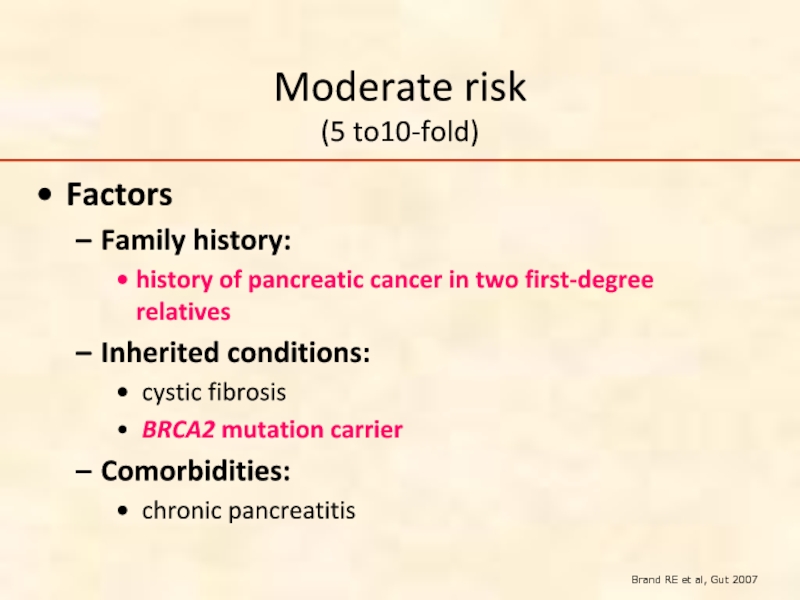
Слайд 42Moderate risk
(5 to10-fold)
Factors
Family history:
history of pancreatic cancer in two first-degree
Inherited conditions:
cystic fibrosis
BRCA2 mutation carrier
Comorbidities:
chronic pancreatitis
Brand RE et al, Gut 2007
Слайд 43High risk
(greater than 10-fold)
Factors
Inherited conditions:
familial atypical multiple mole melanoma
hereditary pancreatitis;
Peutz–Jeghers syndrome;
BRCA2 or BRCA1 mutation carrier with at least one case of pancreatic cancer in first-degree or second-degree relative.
Family history:
three or more first-degree, second-degree or third-degree relatives with pancreatic cancer.
Brand RE et al, Gut 2007
Слайд 44BRCA1/2 in pancreatic cancer
RAMABM HCC
For the 1st degree relative -
High
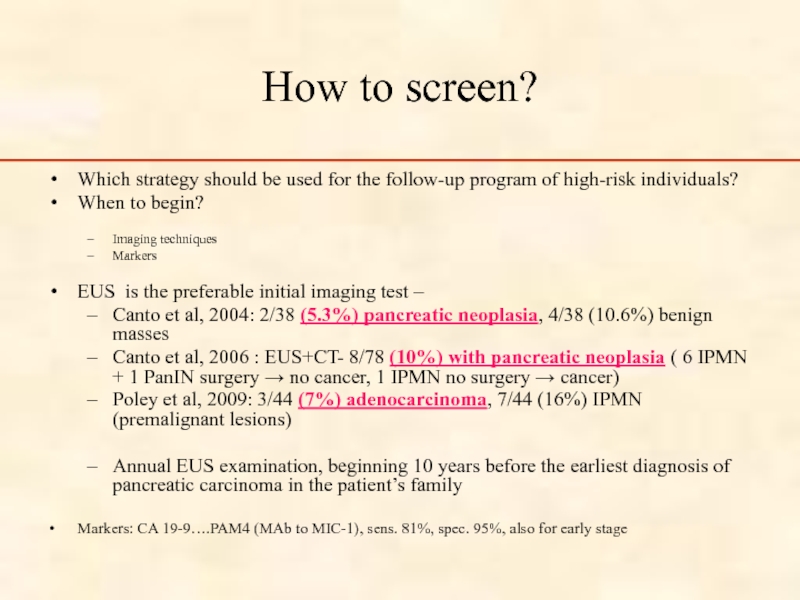
Слайд 45How to screen?
Which strategy should be used for the follow-up
When to begin?
Imaging techniques
Markers
EUS is the preferable initial imaging test –
Canto et al, 2004: 2/38 (5.3%) pancreatic neoplasia, 4/38 (10.6%) benign masses
Canto et al, 2006 : EUS+CT- 8/78 (10%) with pancreatic neoplasia ( 6 IPMN + 1 PanIN surgery → no cancer, 1 IPMN no surgery → cancer)
Poley et al, 2009: 3/44 (7%) adenocarcinoma, 7/44 (16%) IPMN (premalignant lesions)
Annual EUS examination, beginning 10 years before the earliest diagnosis of pancreatic carcinoma in the patient’s family
Markers: CA 19-9….PAM4 (MAb to MIC-1), sens. 81%, spec. 95%, also for early stage
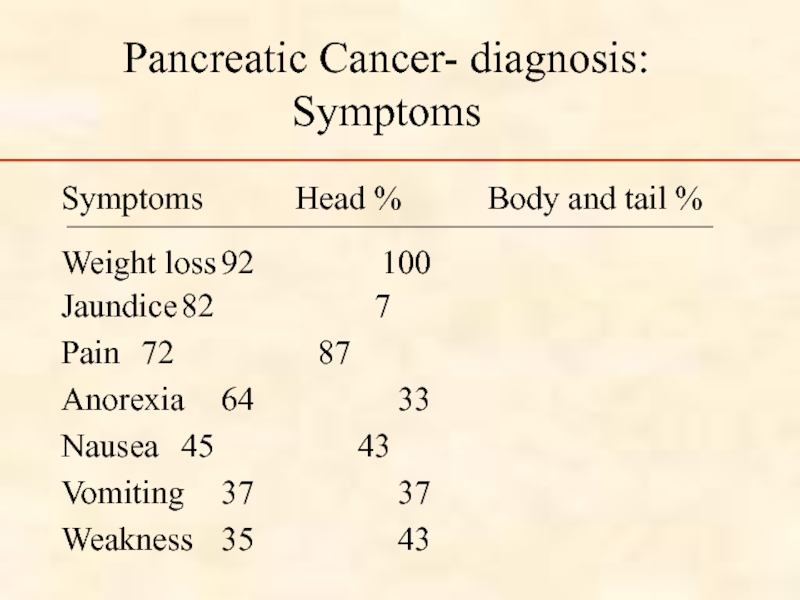
Слайд 47Pancreatic Cancer- diagnosis: Symptoms
Symptoms Head %
Weight loss 92 100
Jaundice 82 7
Pain 72 87
Anorexia 64 33
Nausea 45 43
Vomiting 37 37
Weakness 35 43
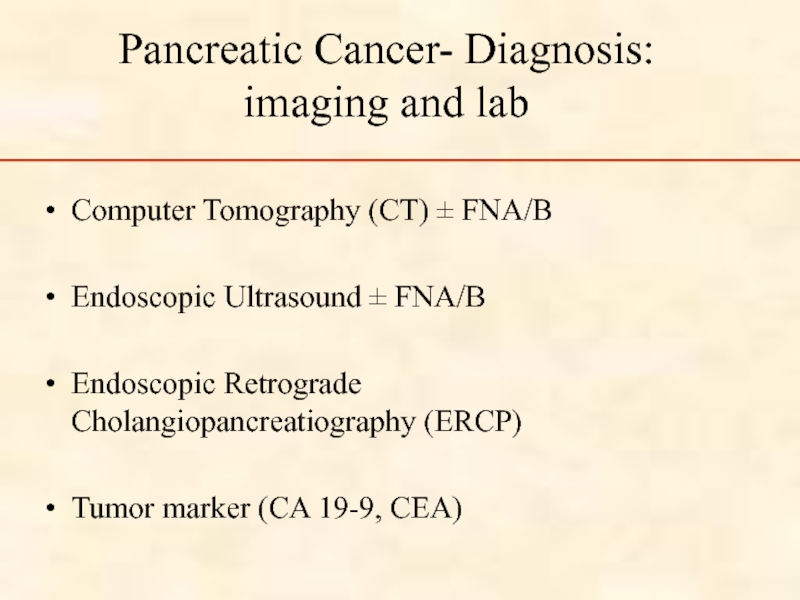
Слайд 48
Pancreatic Cancer- Diagnosis:
imaging and lab
Computer Tomography (CT) ± FNA/B
Endoscopic Ultrasound ±
Endoscopic Retrograde Cholangiopancreatiography (ERCP)
Tumor marker (CA 19-9, CEA)
Слайд 49Staging
Tram et al. “Diagnosis, Staging, and Surveillance of Pancreatic Cancer .”
Слайд 52Pancreatic cancer: stage at diagnosis
10 - 15 % have disease confined
40 % have locally advanced disease = unresectable.
40 – 50% present with visceral metastasis (usually liver)
Слайд 53Pancreatic cancer
Survival
Median
Resectable 15-19 5-20
Locally advanced 6-10 0 - ?
Metastatic 3- 6 0
Слайд 54
Why are the results so poor ?
Symptoms tend to occur rather
Surgery to remove pancreatic cancer is very complicated
The biology of pancreatic cancer makes it an unusually aggressive cancer (small tumor-big effect; resistance to treatment)
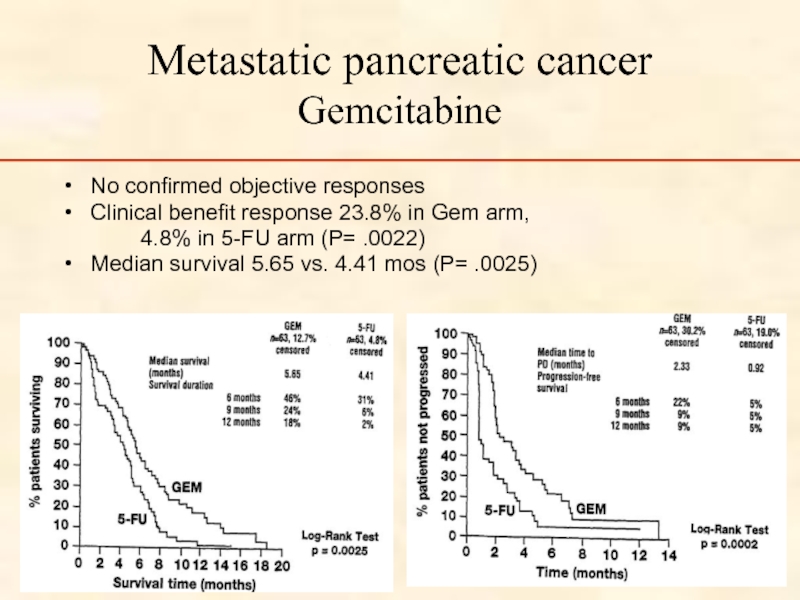
Слайд 58Metastatic pancreatic cancer Gemcitabine
No confirmed objective responses
Clinical benefit response 23.8% in
4.8% in 5-FU arm (P= .0022)
Median survival 5.65 vs. 4.41 mos (P= .0025)
Слайд 59Beyond single-agent gemcitabine ?
Gemcitabine-based combination CT
G + cisplatin
G + capecitabine (xeloda)
G + Abraxane
non-gemcitabine based combination CT
FOLFORINOX (5FU, oxaliplatin, irinotecan) RR X3 (31.6 vs 9.4%), OS 6.8 ↑to 11.1 m
Targeted therapy
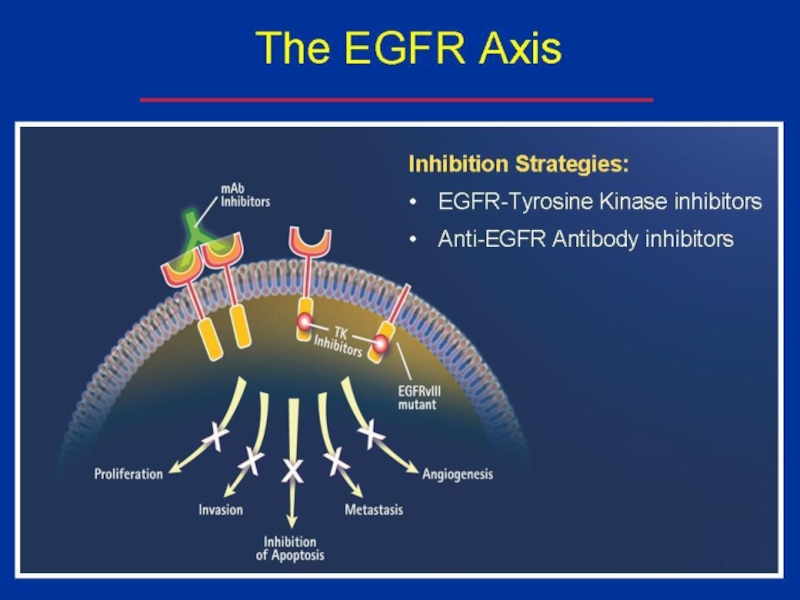
G + erlotinib (tarceva= Human Epidermal Growth Factor Receptor Type 1/Epidermal Growth Factor Receptor (HER1/EGFR) tyrosine kinase inhibitor)
Слайд 600
0,25
0,5
0,75
1
0
6
12
18
24
30
36
HR = 0,57 ; IC95 : 0,45-0,73
p < 0,0001
M
171
171
89
116
28
62
7
20
3
9
2
3
2
2
Gemcitabine
OS = 6.8m
FOLFIRINOX
OS
Probability
Gemcitabine
FOLFIRINOX
ASCO 2010 - Conroy T et al., abstr. 4010
FOLFIRINOX versus gemcitabine
OS
Слайд 61Beyond single-agent gemcitabine ?
Gemcitabine-based combination CT
G + cisplatin
G + capecitabine (xeloda)
non-gemcitabine based combination CT
FOLFORINOX (5FU, oxaliplatin, irinotecan) RR X3, OS 6.8 ↑to 11.1 m
Targeted therapy
G + erlotinib (tarceva= Human Epidermal Growth Factor Receptor Type 1/Epidermal Growth Factor Receptor (HER1/EGFR) tyrosine kinase inhibitor)
Слайд 63GEM plus Erlotinib
6.24 months (GEM+ERL) vs. 5.91 months (GEM)
P=0.038
OS
vs.
Placebo
Pts=569 (naïve advanced pancreatic cancer)
Gemcitabine (1000 mg/m2) +
Erlotinib (100 or 150 mg/die)
Слайд 65Locally advanced disease (LAD)
clinical highlights
Median survival of LAD is 6-10 months
Most
Слайд 66LAD
Aims of treatment
Improvement of quality of life = clinical benefit response
Local control = prolongation of survival ?
Downstaging = resectability ?
Слайд 67Practical guidelines 2013
Rambam
Gemcitabine-based chemotherapy for up to 4 months (as long
Single-agent gemcitabine in patients with poor performance status.
Слайд 70Resectable pancreatic cancer
Long-term survival after resection (10-20% 5-y),
Local recurrence (50-85%), peritoneal spread (40%), liver metastases (60-90%).
Do we have an effective adjuvant therapy?
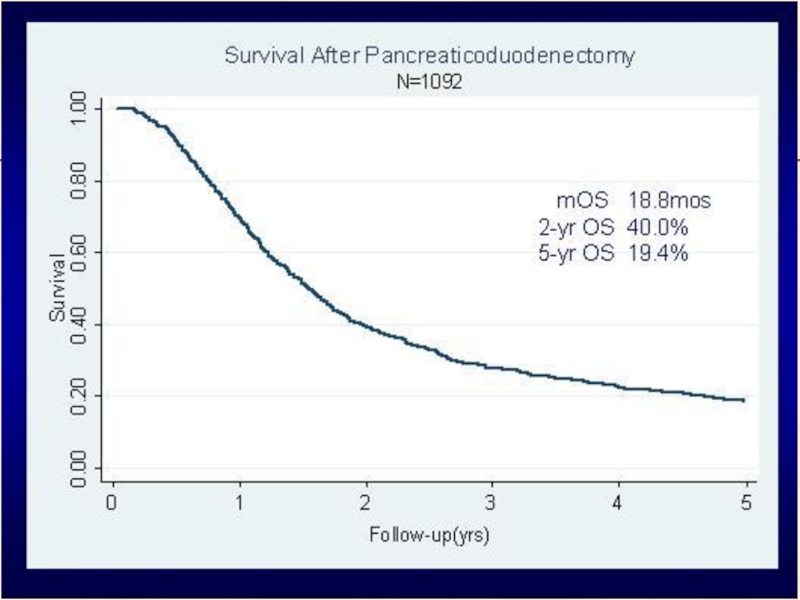
Слайд 71overall survival among all 1,092 resected pancreatic adenocarcinoma patients with (583,
Median OS
S = 15.5 m
▲ 5.6 m
S+CRT= 21.1 m
2 y OS
S = 34.6%
▲ 10.1%
S+CRT = 44.7%
5 y OS
S = 16.1%
▲ 6.2%
S+CRT = 22.3%
Charles C. Hsu et al. Ann Surg Oncol. 2010 April; 17(4): 981–990.
Слайд 72Adjuvant chemoradiotherapy –
randomized studies (2)
ESPAC-1 (European Study Group for Pancreatic Cancer)
Neoptolemos, LANCET 2001 + NEJM 2004 (median FU=47m)
CT/RT (split-course 40 Gy + bolus 5FU daily for 3 initial days of RT)
vs.
CT ( 5FU + folinic acid, Mayo x 6 cycles)
vs
CT/RT+CT
vs.
Observation
Слайд 74Resectable pancreatic cancer
adjuvant therapy
chemotherapy only?
Charité Onkologie [CONKO]-001)
German study
(Oettle, JAMA 2007)
(Neuhaus, ASCO
DFS-m OS-m
(189 pts): Gemcitabine (6 m) 13.4 22.8
(182 pts): observation 6.9 20.2 p<0.001 p=0.005
(cross over !)
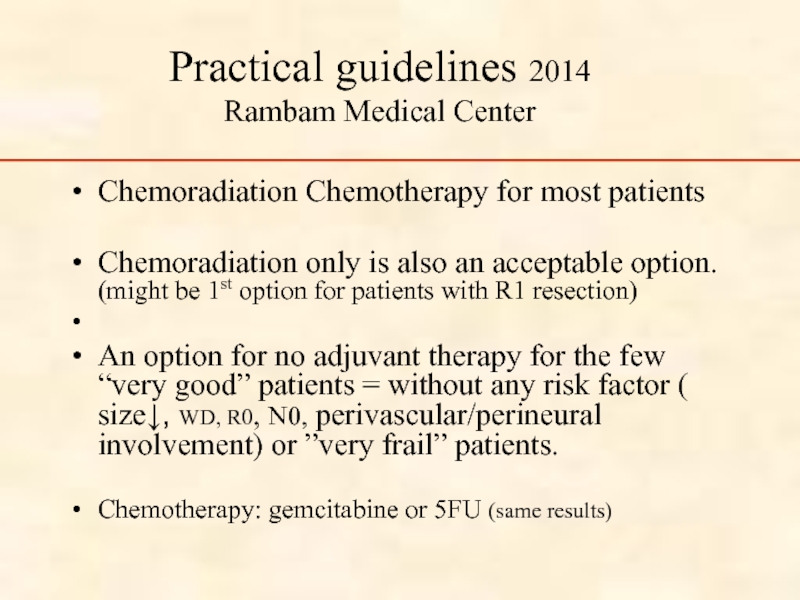
Слайд 76Practical guidelines 2014
Rambam Medical Center
Chemoradiation Chemotherapy for most patients
Chemoradiation only is
An option for no adjuvant therapy for the few “very good” patients = without any risk factor ( size↓, WD, R0, N0, perivascular/perineural involvement) or ”very frail” patients.
Chemotherapy: gemcitabine or 5FU (same results)
Слайд 77Still unclear…
Pancreatology. 2012 Mar;12(2):162-9. Epub 2012 Feb
Adjuvant chemotherapy, with or
Ren F, Xu YC, Wang HX, Tang L, Ma Y.
Department of Oncology, Shanghai Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200127, China.
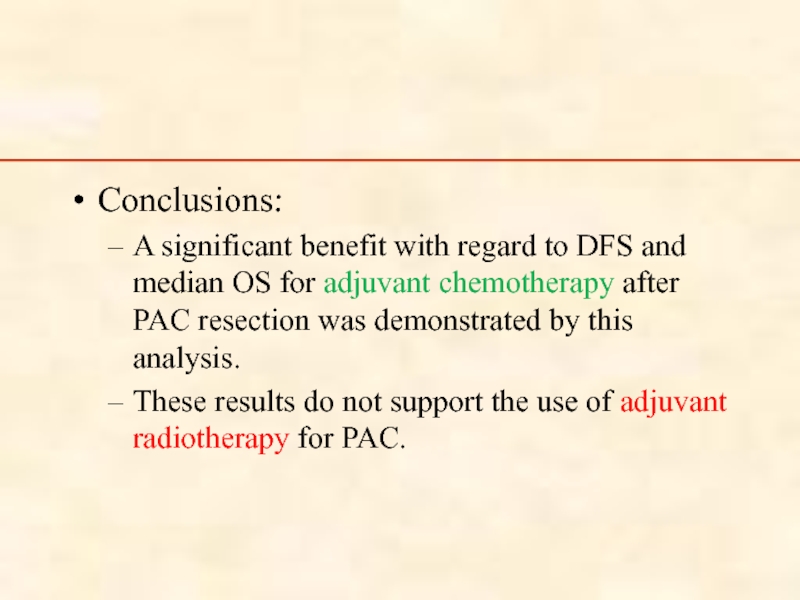
Слайд 78
Conclusions:
A significant benefit with regard to DFS and median OS for
These results do not support the use of adjuvant radiotherapy for PAC.
Слайд 80Personalized medicine
patients with the same cancer type respond differently to therapies
Acquired or germeline changes in our DNA that cause a cancer to develop and grow can differ from person to person with the same tumor.
Molecular testing reveals those differences.
Слайд 81Personalized medicine
Gene expression profiling, molecular profiling, of the specific tumor of
To find biomarkers with ↑, ↓, mutated genes = potential targets for different drugs
Metabolism
Direct targeting
Слайд 82RRM1 → Gemcitabine
RRM1 (Ribonucleotide Reductase subunit M1) -involved in DNA
Thus, RRM1 gene-over-expression may be a negative predictive marker for treatment with gemcitabine.
Слайд 83
SPARC (Secreted Protein Acidic and Rich in Cystein) is a matrix-associated
Because of a SPARC-albumin interaction, tumoral SPARC facilitates the accumulation of albumin in the tumor and increases the effectiveness of albumin-bound drugs
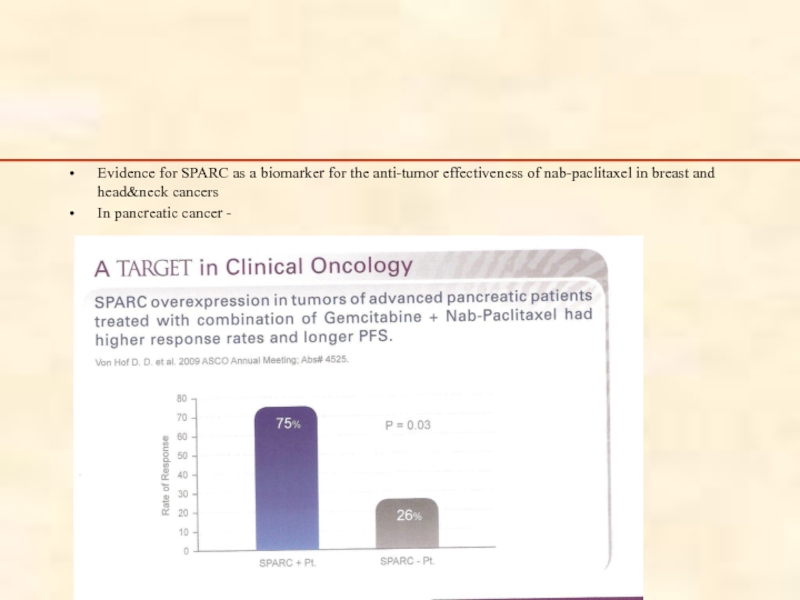
Слайд 85
Evidence for SPARC as a biomarker for the anti-tumor effectiveness of
In pancreatic cancer -
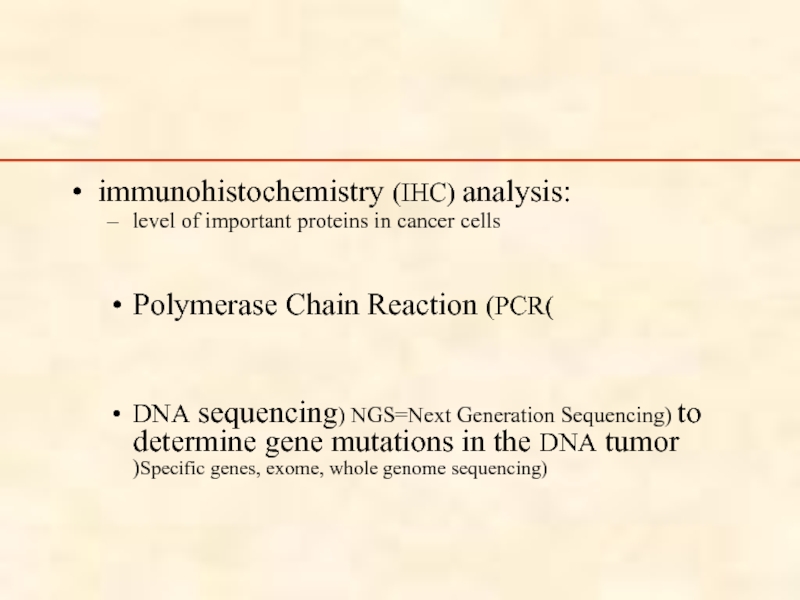
Слайд 87
immunohistochemistry (IHC) analysis:
level of important proteins in cancer cells
Polymerase Chain Reaction
DNA sequencing) NGS=Next Generation Sequencing) to determine gene mutations in the DNA tumor )Specific genes, exome, whole genome sequencing)
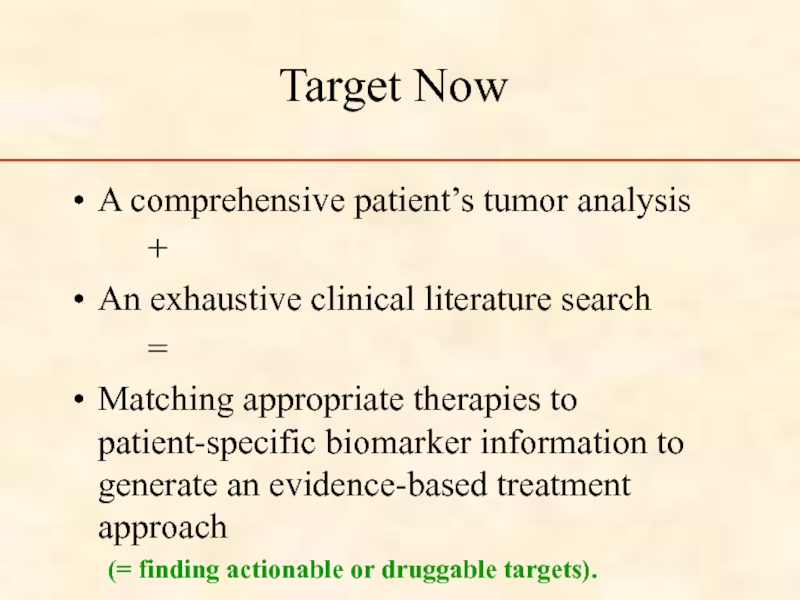
Слайд 88Target Now
A comprehensive patient’s tumor analysis
+
An exhaustive clinical literature search
=
Matching appropriate
(= finding actionable or druggable targets).
Слайд 89A Retrospective Investigation to Evaluate the Use of Target Now® Assay
The aim of the investigation was to retrospectively study the data from locally advanced and metastatic pancreatic cancer patients who have had their tumor profiled using the Target Now® commercial assay.
They all received at least one treatment line for advanced pancreatic cancer prior to TN-directed therapy.
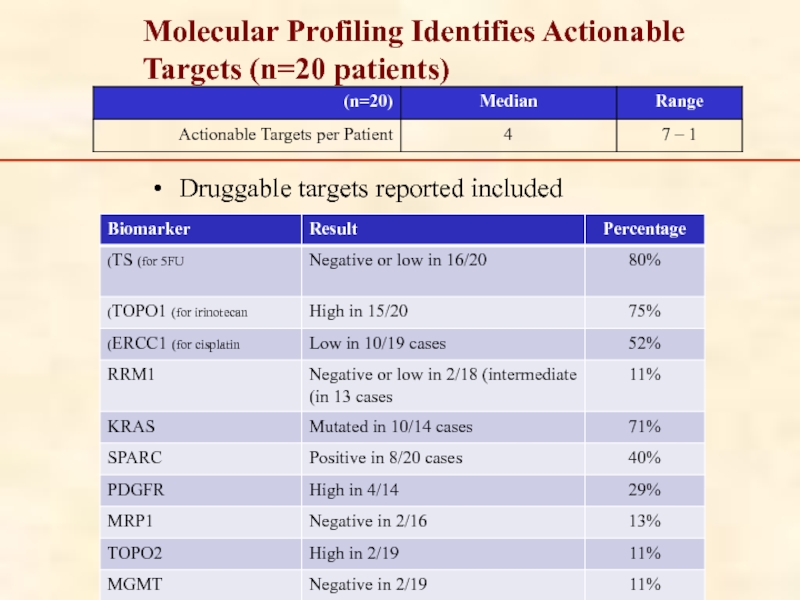
Слайд 90Druggable targets reported included
Molecular Profiling Identifies Actionable Targets (n=20 patients)
Слайд 91Molecular Profiling Identifies Potential Therapeutic Options in Advanced Pancreatic Cancer (n=20
Слайд 92 Molecular Profiling Guided Treatment Choices (n=20 patients)
The graph above shows
which were used alone or in combination in all lines (32) administered following receipt
of the molecular profiling information (1-4 lines per pt, median:1)
Слайд 93Nab-paclitaxel
Capecitabine
+ Irinotecan
Capecitabine
Gemcitabine
+Oxaliplatin
Nab-paclitaxel
Capecitabine
Capecitabine
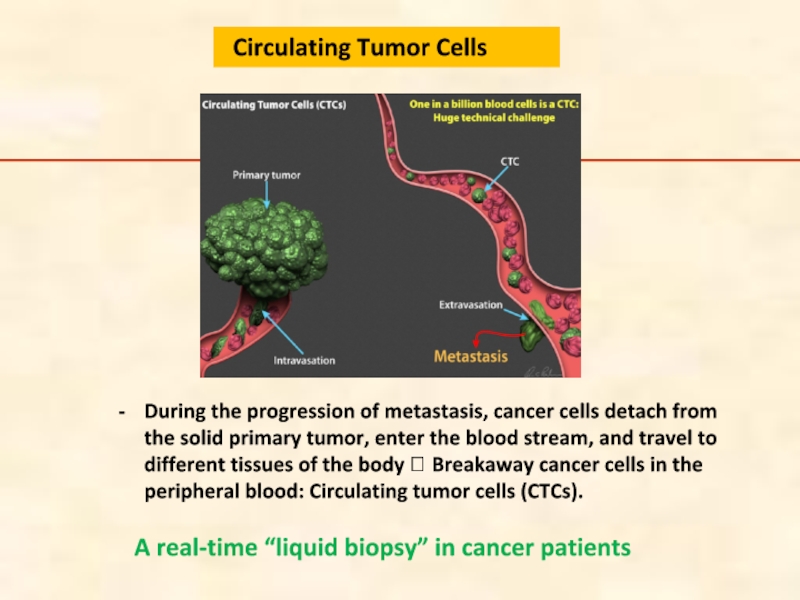
Слайд 94During the progression of metastasis, cancer cells detach from the solid
A real-time “liquid biopsy” in cancer patients
Circulating Tumor Cells
Слайд 96M Murtaza et al. Nature April 7 (2013), Cambridge, UK
Identification of
exome sequencing of serial plasma samples (= circulating cell-free tumor DNA)
Nineteen samples in 5 pts with breast, lung, ovarian cancers



















































![Resectable pancreatic cancer adjuvant therapy chemotherapy only?Charité Onkologie [CONKO]-001) German study (Oettle, JAMA 2007) (Neuhaus, ASCO 2008)](/img/tmb/5/498826/0c01508e6b68c494bafa9d893f8ad1f8-800x.jpg)