- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Molecular diffusion презентация
Содержание
- 1. Molecular diffusion
- 2. 2.1 FICK’S LAW
- 3. Where, JA is the molar flux of
- 4. 2.2 Diffusion coefficient
- 5. Diffusivity decreases with
- 6. The diffusivity increases with increase
- 7. Diffusivity of gas, liquid, and solid
- 9. Three models of diffusion process in gas,
- 10. 2.3 Ratio between heat and molecular diffusivity
- 11. 2.3 Measurement of gas-phase diffusion coefficient (a) Twin-bulb method
- 12. Two bulbs are
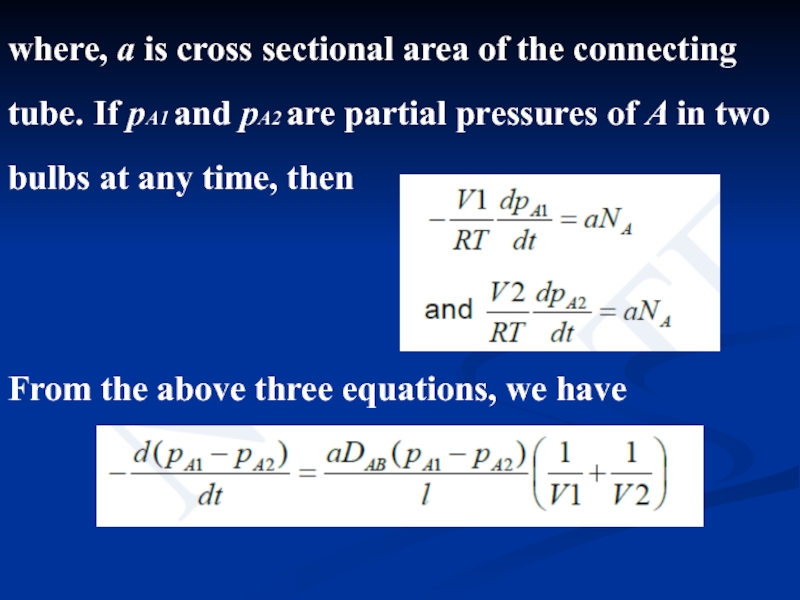
- 13. where, a is cross sectional area of
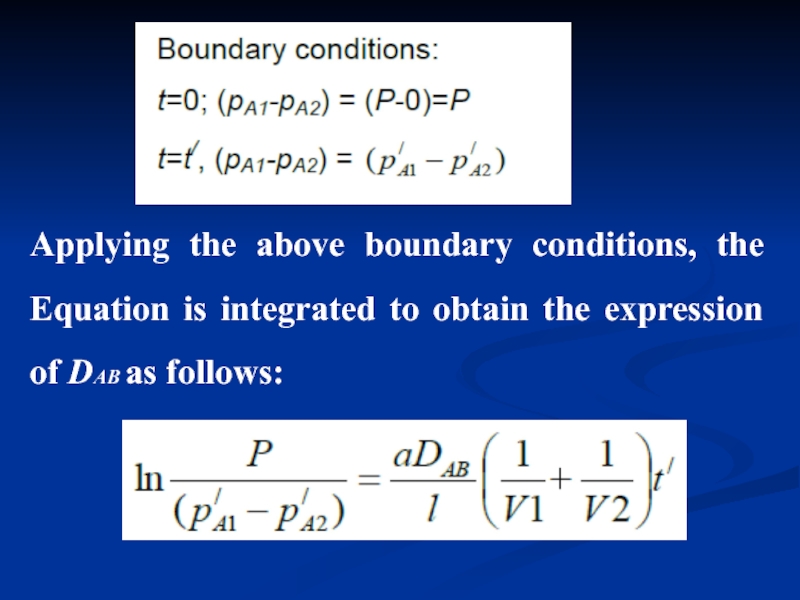
- 14. Applying the above boundary conditions, the Equation
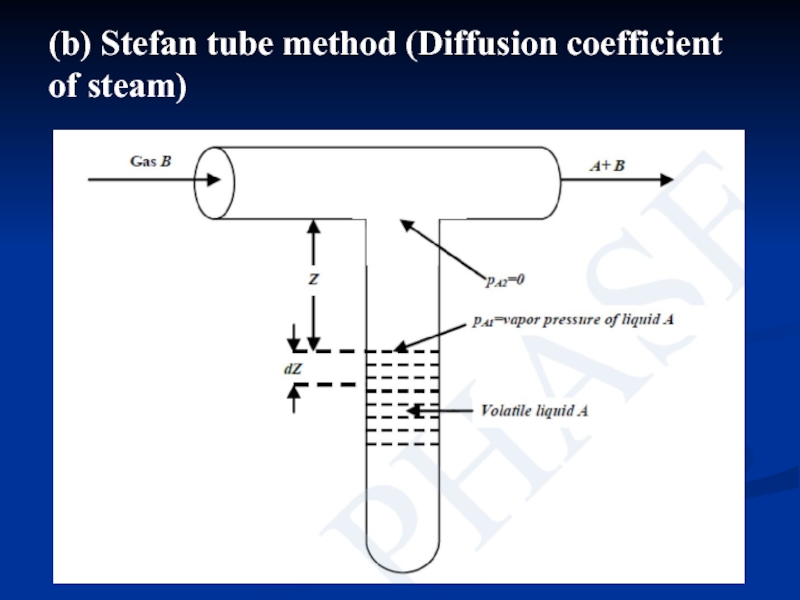
- 15. (b) Stefan tube method (Diffusion coefficient of steam)
- 16. Stefan tube consists
- 17. where, partial pressure of A at liquid
- 18. 2.4 Estimation of gas diffusion coefficient where,
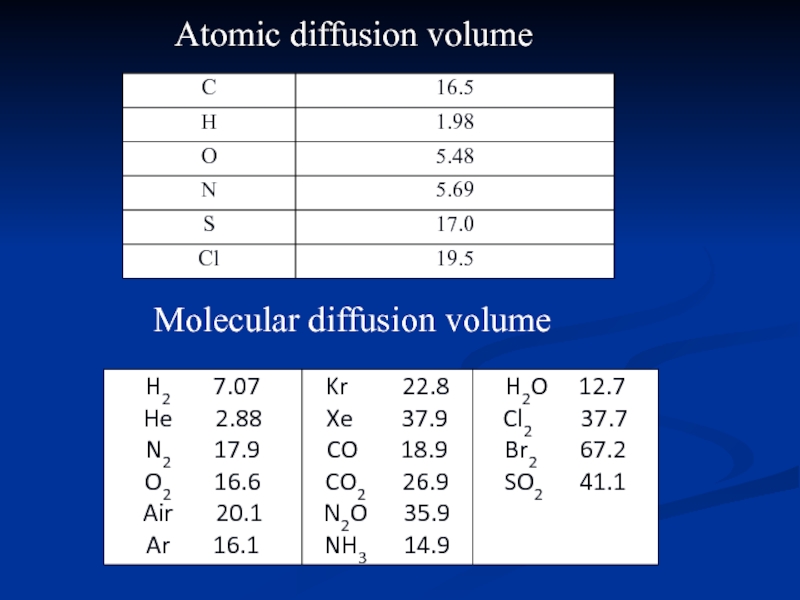
- 19. Atomic diffusion volume Molecular diffusion volume
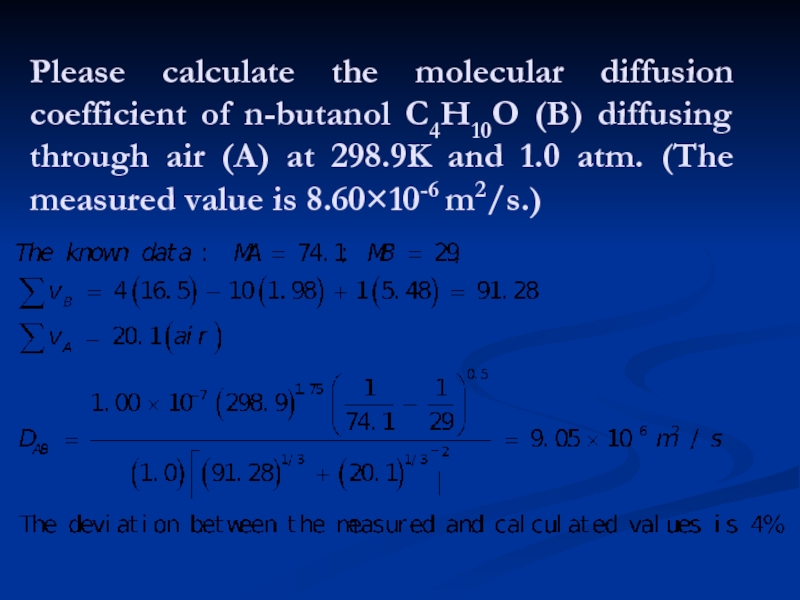
- 20. Please calculate the molecular diffusion coefficient of
- 21. Stokes-Einstein Equation Liquid diffusivity varies linearly
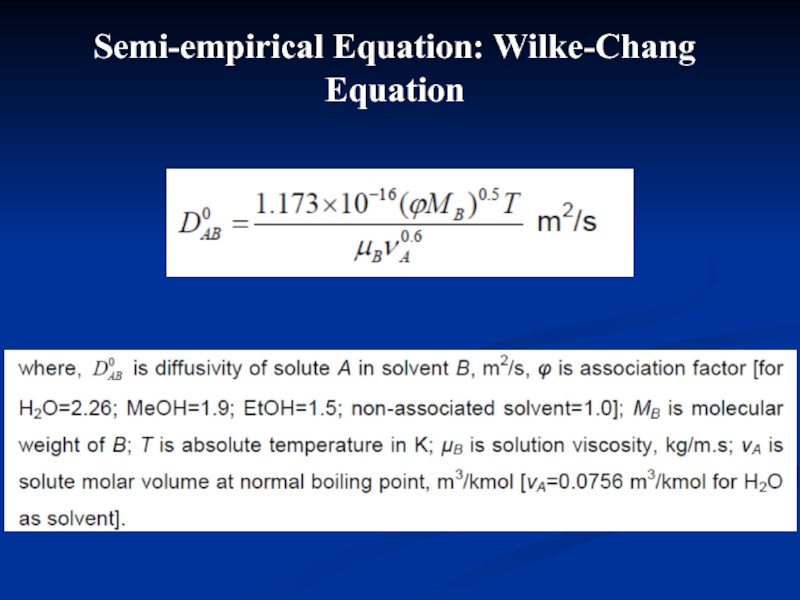
- 22. Semi-empirical Equation: Wilke-Chang Equation
- 23. 2.6 Diffusion in porous media Porous materials
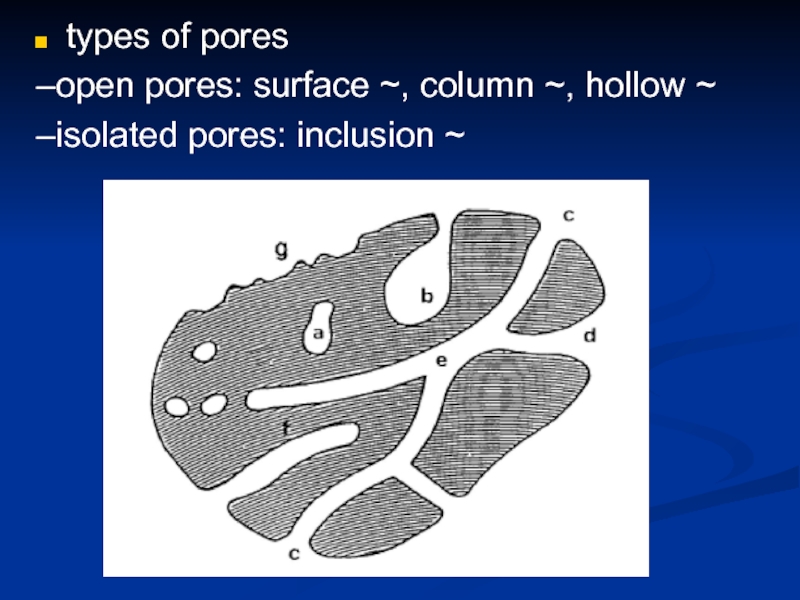
- 24. types of pores –open pores: surface
- 25. Pore size: (generally pore width): the distance
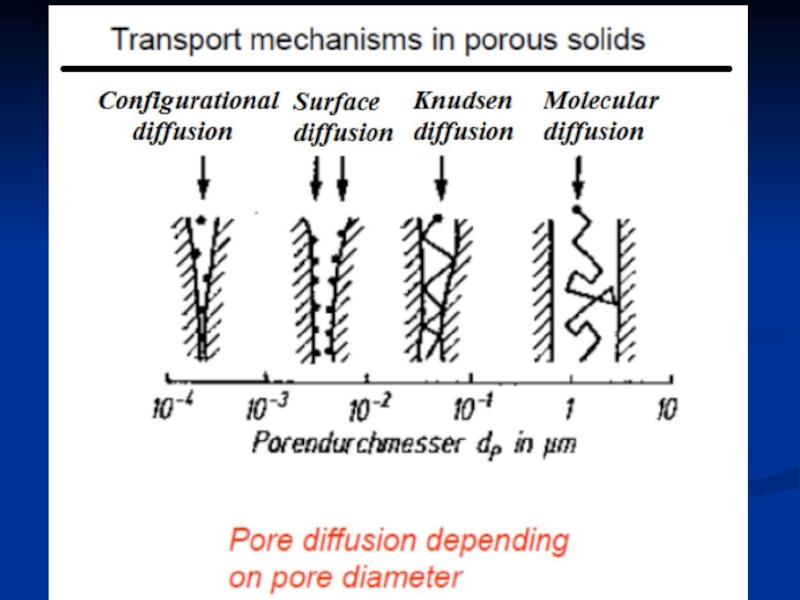
- 26. Diffusion phenomena in porous solids Molecular
- 28. Molecular diffusion (Collision principle) The probability of
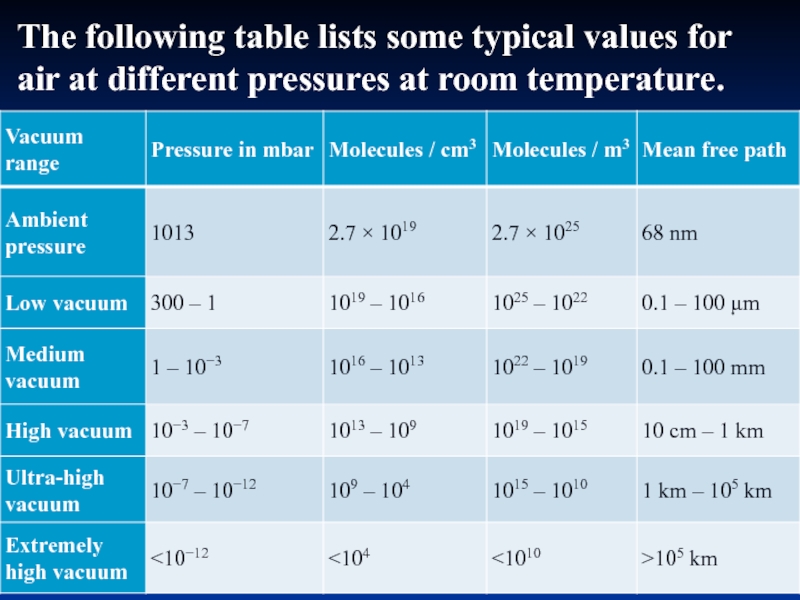
- 29. The following table lists some typical values for air at different pressures at room temperature.
- 30. Knudsen diffusion (Collision principle)
- 31. Configurational diffusion –pore diameter within molecular
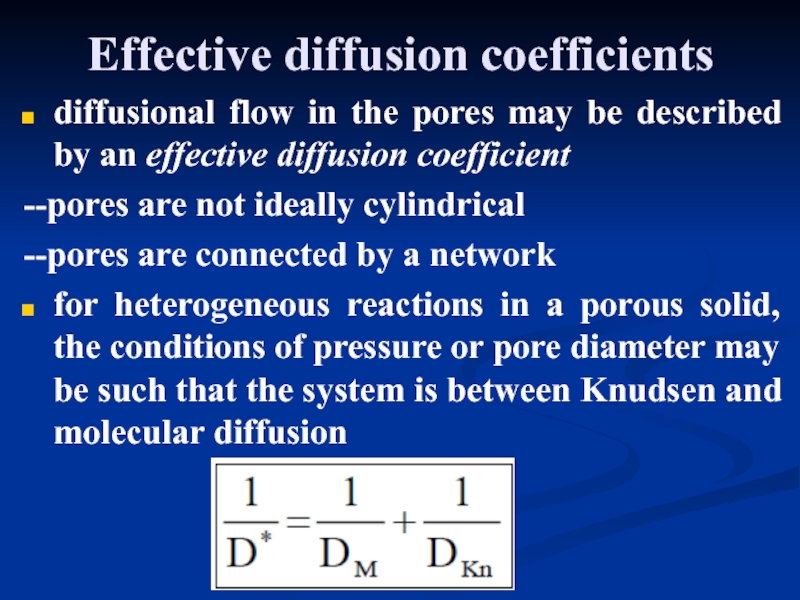
- 32. Effective diffusion coefficients diffusional flow
Слайд 2 2.1 FICK’S LAW
Adolf Fick in
Слайд 3Where, JA is the molar flux of component A in the
Слайд 42.2 Diffusion coefficient
The proportionality factor of Fick’s law
Слайд 5 Diffusivity decreases with increase in pressure (DAB∝1/p
Relationship between diffusivity and pressure
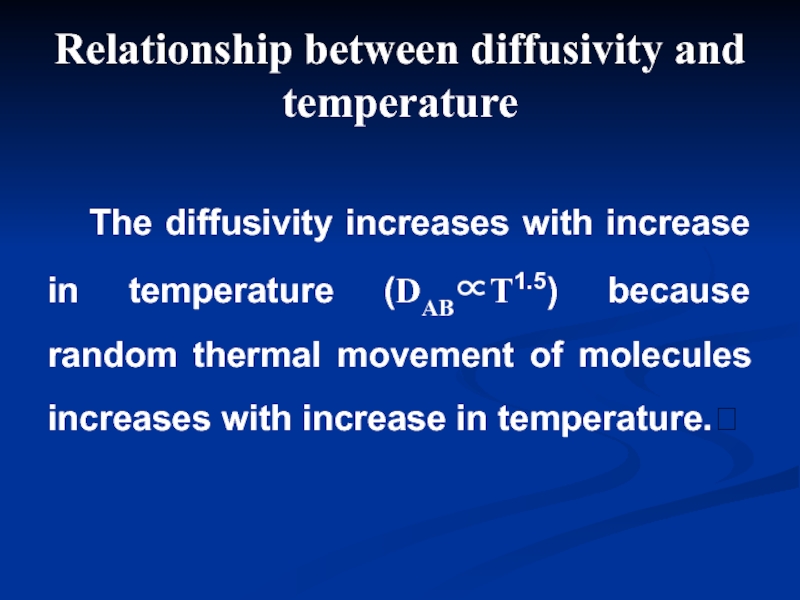
Слайд 6 The diffusivity increases with increase in temperature (DAB∝T1.5) because
Relationship between diffusivity and temperature
Слайд 7Diffusivity of gas, liquid, and solid
The
Слайд 8 Diffusion is almost impossible
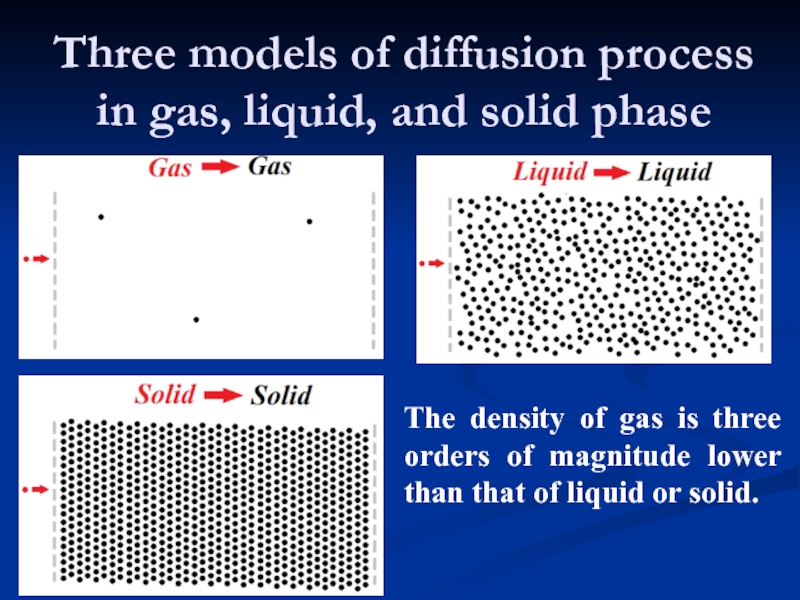
Слайд 9Three models of diffusion process in gas, liquid, and solid phase
The
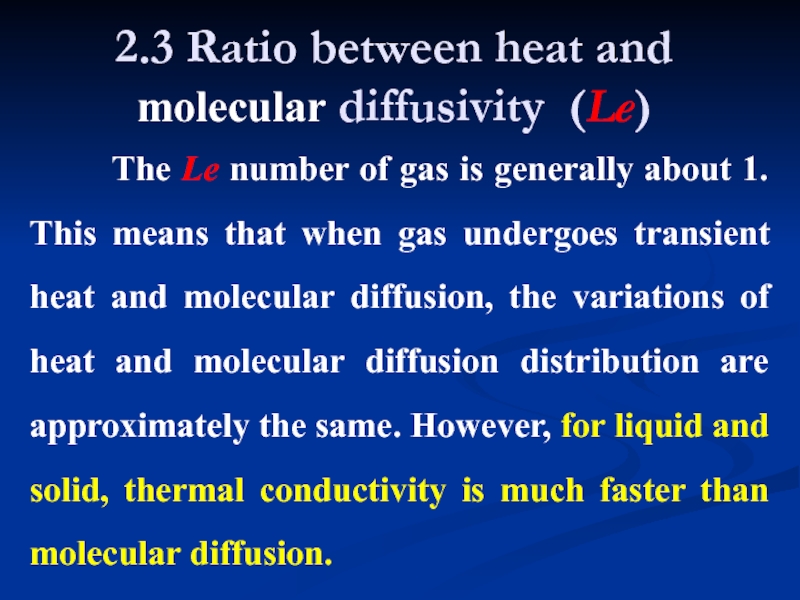
Слайд 102.3 Ratio between heat and molecular diffusivity (Le)
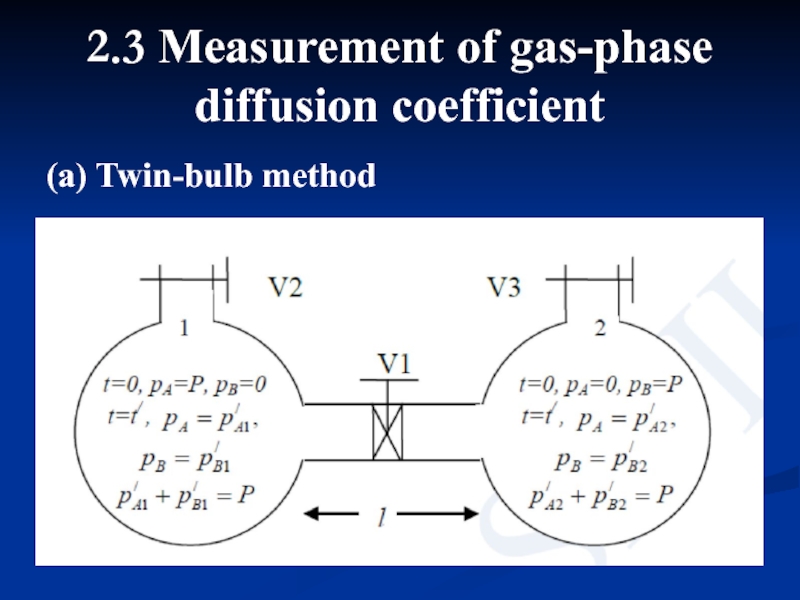
Слайд 12 Two bulbs are connected by a narrow
Слайд 13where, a is cross sectional area of the connecting tube. If
From the above three equations, we have
Слайд 14Applying the above boundary conditions, the Equation is integrated to obtain
Слайд 16 Stefan tube consists of a T-tube, placed
Слайд 17where, partial pressure of A at liquid surface, pA1 is equal
Слайд 182.4 Estimation of gas diffusion coefficient
where, T is temperature in K
P is total pressure in bar
νA, νB are atomic diffusion volume in m3.
Слайд 20Please calculate the molecular diffusion coefficient of n-butanol C4H10O (B) diffusing
Слайд 21Stokes-Einstein Equation
Liquid diffusivity varies linearly with absolute temperature and inversely
2.5 Estimation of liquid-phase diffusion coefficient
Слайд 232.6 Diffusion in porous media
Porous materials in nature and industry: sand
main feature: cavities in a solid matrix, cavities are partly or fully connected, and accessible for probe molecules.
porosities are often desired and of importance in medicine, membranes, sorbents, ceramics, and catalysts.
Слайд 25Pore size: (generally pore width): the distance between two opposite walls
–Micropores (< 2 nm)
–Mesopores (2-50 nm)
–Macropores (> 50 nm)
Слайд 26Diffusion phenomena in porous solids
Molecular diffusion
Knudsen diffusion
Surface diffusion
–not of technical importance
Configurational diffusion
–pore diameter within molecular dimensions (0.3-1 nm) as for zeolites
–diffusion coefficients are smaller by some orders of magnitude
Слайд 28Molecular diffusion (Collision principle)
The probability of collision between molecules and molecules
In physics, the mean free path is the average distance traveled by a moving particle (such as an atom, a molecule, a photon) between successive impacts (collisions), which modifies its direction or energy or other particle properties..
Слайд 29The following table lists some typical values for air at different
Слайд 30Knudsen diffusion (Collision principle)
Surface diffusion (Adsorption principle)
Adsorption balance is established in
Слайд 31Configurational diffusion
–pore diameter within molecular dimensions (0.3-1 nm) as for
–diffusion coefficients are smaller by some orders of magnitude
Слайд 32
Effective diffusion coefficients
diffusional flow in the pores may be described
--pores are not ideally cylindrical
--pores are connected by a network
for heterogeneous reactions in a porous solid, the conditions of pressure or pore diameter may be such that the system is between Knudsen and molecular diffusion