- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Guidelines in Rheumatology презентация
Содержание
- 1. Guidelines in Rheumatology
- 2. Genetic Predisposition for Development of Ankylosing Spondylitis
- 3. Natural History of AS Highly variable Early
- 4. Burden of Illness Functional disability Potential complications
- 5. Obstacles to Desirable Outcomes in AS Until
- 6. Advances in Medicine: Hope for Patients With
- 7. Pathogenesis of AS Incompletely understood, but knowledge
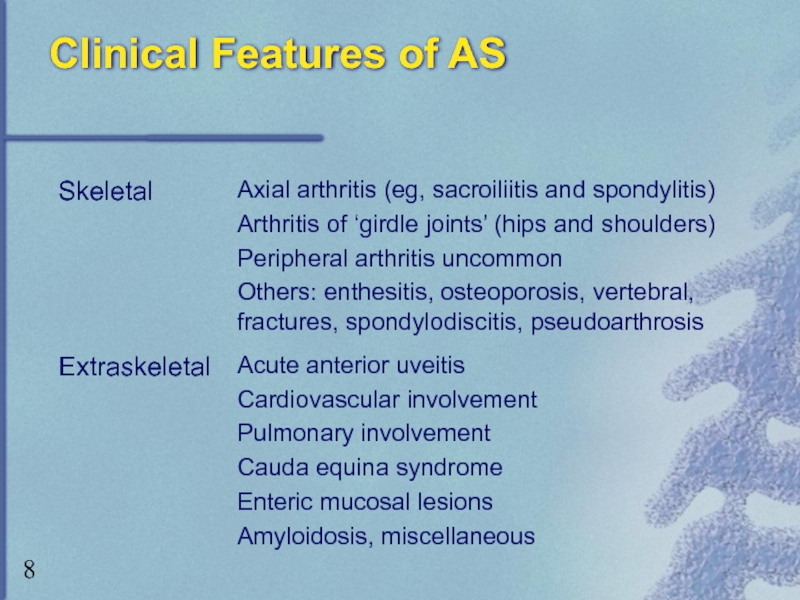
- 8. Clinical Features of AS
- 9. Modified New York Criteria for the Diagnosis
- 10. Disease Activity Assessment BASFI = Bath Ankylosing
- 11. Bath Ankylosing Spondylitis Functional Index (BASFI) Visual
- 12. Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)
- 13. ASsessment in Ankylosing Spondylitis (ASAS) ASAS 20:
- 14. Introduction of Anti-TNF Agents for the
- 15. Tumor Necrosis Factor: Functions of the Proinflammatory
- 16. Pathogenesis of Joint Destruction Bone
- 17. US Modifications of the ASAS International Guidelines:
- 18. Contraindications for Anti-TNF Therapy Current or
- 19. Monitoring and Discontinuing Treatment With Anti-TNF Agents
- 20. Anti-TNF Agents Etanercept Approved in the United
- 21. Etanercept Vs. Infliximab: Pharmacologic Characteristics
- 22. Etanercept vs Infliximab: Clinical Differences Etanercept Approved
- 23. Etanercept for the Treatment of AS: Clinical
- 24. Etanercept for the Treatment of AS: Clinical
- 25. Etanercept: Adverse Events *P
- 26. Etanercept: Adverse Events (cont) Serious infections and
- 27. Infliximab for the Treatment of AS: Clinical
- 28. Infliximab for the Treatment of AS: Clinical
- 29. Infliximab: Adverse Events * Approximation based on all clinical studies
- 30. Infliximab: Adverse Events (cont) Serious infections and
- 31. Anti-TNF Agents: Summary Anti-TNF agents target underlying
- 32. AS Treatment Algorithm: Patients with Axial AS
- 33. AS Treatment Algorithm: Patients with Predominantly Symptomatic
Слайд 2Genetic Predisposition for Development of Ankylosing Spondylitis (AS)
AS and HLA-B27 –
Ethnic and racial variability in presence and expression of HLA-B27
Слайд 3Natural History of AS
Highly variable
Early stages: spontaneous remissions and exacerbations
Spectrum of
Mild with limited sacroiliac or lumbar joint involvement to severe, debilitating disease
“Pre-spondylitic” phase – unrecognized period of progressive structural damage over a 5-to-10-year period
Average delay in diagnosis is 8.9 years
Слайд 4Burden of Illness
Functional disability
Potential complications
Quality-of-life issues
Pain, stiffness, fatigue, sleep problems
Healthcare costs
75% indirect medical costs
Missed workdays
Limited-activity days
Слайд 5Obstacles to Desirable Outcomes in AS Until Recently
Diagnostic and classification limitations
Lack
Until recently, limited treatment options
NSAIDs, COX-2 inhibitors, DMARDs
Mostly symptomatic relief only
Minimal impact on natural course of disease
Слайд 6Advances in Medicine:
Hope for Patients With AS
Increased understanding of pathophysiologic processes
Advent of Anti-TNF agents
International meetings by ASAS (ASsessment in AS working group) to address need for universal standards
Development of ASAS guidelines
US modifications to the ASAS International Guidelines to meet realities of clinical practice in the United States
Слайд 7Pathogenesis of AS
Incompletely understood, but knowledge increasing
Interaction between HLA-B27 and T-cell
Increased concentration of T-cells, macrophages, and proinflammatory cytokines
Role of TNF
Inflammatory reactions ? produce hallmarks of disease
Слайд 9Modified New York Criteria for the Diagnosis of AS
Clinical Criteria
Low back
Limitation of lumbar spine motion, sagittal and frontal planes
Limitation of chest expansion relative to normal values for age and sex
Radiologic Criteria
Sacroiliitis grade ≥ 2 bilaterally or grade 3 – 4 unilaterally
Grading
Definite AS if radiologic criterion present plus at least one clinical criteria
Probable AS if:
Three clinical criterion
Radiologic criterion present, but no signs or symptoms satisfy clinical criteria
Слайд 10Disease Activity Assessment
BASFI = Bath Ankylosing Spondylitis Functional Index
BASDAI = Bath
ASAS - IC = ASsessment in Ankylosing Spondylitis Improvement Criteria
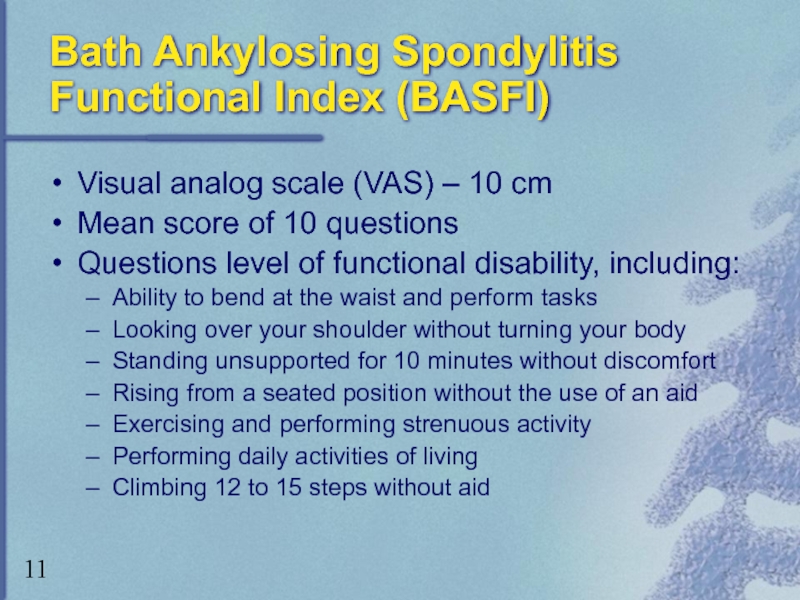
Слайд 11Bath Ankylosing Spondylitis Functional Index (BASFI)
Visual analog scale (VAS) – 10
Mean score of 10 questions
Questions level of functional disability, including:
Ability to bend at the waist and perform tasks
Looking over your shoulder without turning your body
Standing unsupported for 10 minutes without discomfort
Rising from a seated position without the use of an aid
Exercising and performing strenuous activity
Performing daily activities of living
Climbing 12 to 15 steps without aid
Слайд 12Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)
A self-administered instrument (using 10-cm
Fatigue/tiredness
AS spinal (back, neck) or hip pain
Pain/swelling in joints other than above
Level of discomfort from tender areas
Morning stiffness from the time you awake
How long does morning stiffness last?
Слайд 13ASsessment in Ankylosing Spondylitis (ASAS)
ASAS 20: An improvement of > 20%
Patient global assessment (by VAS global assessment)
Pain assessment (the average of VAS total and nocturnal pain scores)
Function (represented by BASFI)
Inflammation (the average of the BASDAI’s last two VAS concerning morning stiffness intensity and duration)
Absence of deterioration in the potential remaining domain
(deterioration is defined as > 20% worsening)
Слайд 14Introduction of Anti-TNF
Agents for the Treatment of Ankylosing Spondylitis
US Modifications
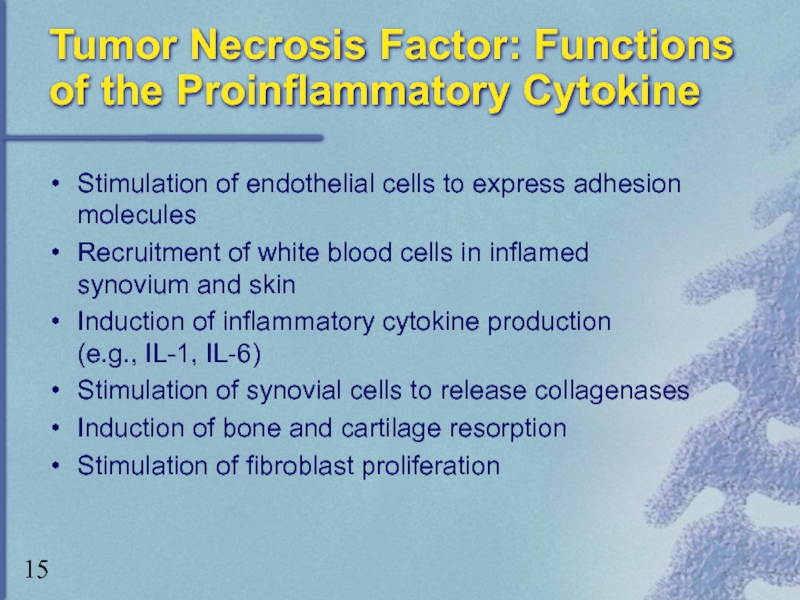
Слайд 15Tumor Necrosis Factor: Functions of the Proinflammatory Cytokine
Stimulation of endothelial cells
Recruitment of white blood cells in inflamed synovium and skin
Induction of inflammatory cytokine production (e.g., IL-1, IL-6)
Stimulation of synovial cells to release collagenases
Induction of bone and cartilage resorption
Stimulation of fibroblast proliferation
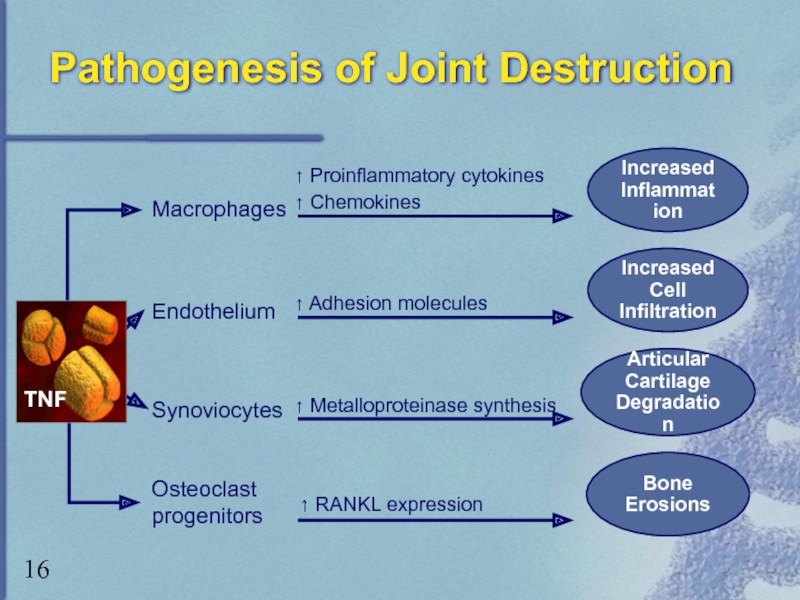
Слайд 16Pathogenesis of Joint Destruction
Bone
Erosions
Macrophages
Endothelium
Synoviocytes
↑ Proinflammatory cytokines
↑ Chemokines
↑ Adhesion molecules
↑
Articular
Cartilage
Degradation
Increased Cell
Infiltration
Increased
Inflammation
Osteoclast
progenitors
↑ RANKL expression
TNF
Слайд 17US Modifications of the ASAS International Guidelines: Appropriate Patients for Anti-TNF
Definitive AS according to Modified New York Criteria
Active disease for ≥ 4 weeks
BASDAI > 4 cm at two times, 1 month apart
Physician Global Assessment ≥ 2 on Likert Scale
Treatment Failures
All types AS – lack of response/intolerability > 2 NSAIDs
for ≥ 3 months
Patients with peripheral arthritis – lack of response/intolerability to > 1 DMARD, sulfasalazine preferred
Слайд 18Contraindications for
Anti-TNF Therapy
Current or recurrent infections
Tuberculosis
Multiple sclerosis
Lupus
Malignancy
Pregnant or lactating
Слайд 19Monitoring and Discontinuing Treatment With Anti-TNF Agents
ASAS core set of outcome
Physical function, pain, spinal mobility, patient’s global assessment, stiffness, peripheral joints and entheses, acute phase reactant, fatigue
Assess at 6 to 8 weeks and discontinue patients who do not meet response criteria
BASDAI: Reduction of ≥ 2 units and
Physician Global Assessment > 1
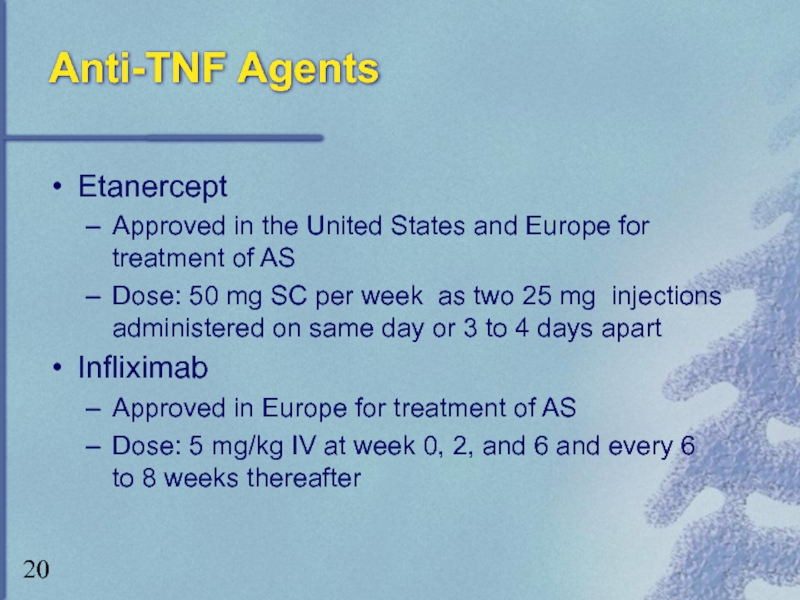
Слайд 20Anti-TNF Agents
Etanercept
Approved in the United States and Europe for treatment of
Dose: 50 mg SC per week as two 25 mg injections administered on same day or 3 to 4 days apart
Infliximab
Approved in Europe for treatment of AS
Dose: 5 mg/kg IV at week 0, 2, and 6 and every 6 to 8 weeks thereafter
Слайд 22Etanercept vs Infliximab:
Clinical Differences
Etanercept
Approved by FDA for treatment of psoriatic arthritis,
Infliximab
Approved by FDA for treatment of Crohn’s disease and rheumatoid arthritis
Safety
Tuberculosis and histoplasmosis
Post-marketing reports and FDA surveillance database indicate disproportionate association between infliximab and risk of such (opportunistic) infections
Слайд 23Etanercept for the Treatment of AS: Clinical Trials
Marzo-Ortega, et al.
Significant
86% MRI-detected entheseal lesions regressed completely or improved
Marzo-Ortega, et al.
Mean hip and spine BMD increased with 24 weeks’ etanercept treatment
Gorman, et al.
80% etanercept-treated patients, 30% placebo-treated patients achieved ASAS 20 at 4 months
6-month extension: 83%, 80%, 60% achieved ASAS 20, ASAS 50, ASAS 70, respectively
95% of patients treated only with etanercept (not placebo) over 10 months achieved ASAS 20
Слайд 24Etanercept for the Treatment of AS: Clinical Trials (cont)
Brandt, et al.
57%
56% in placebo group improved following switch to etanercept
Improvements ceased once etanercept therapy was discontinued
Davis, et al.
57% etanercept-treated patients and 22% placebo-treated patients achieved ASAS 20 at 24 weeks
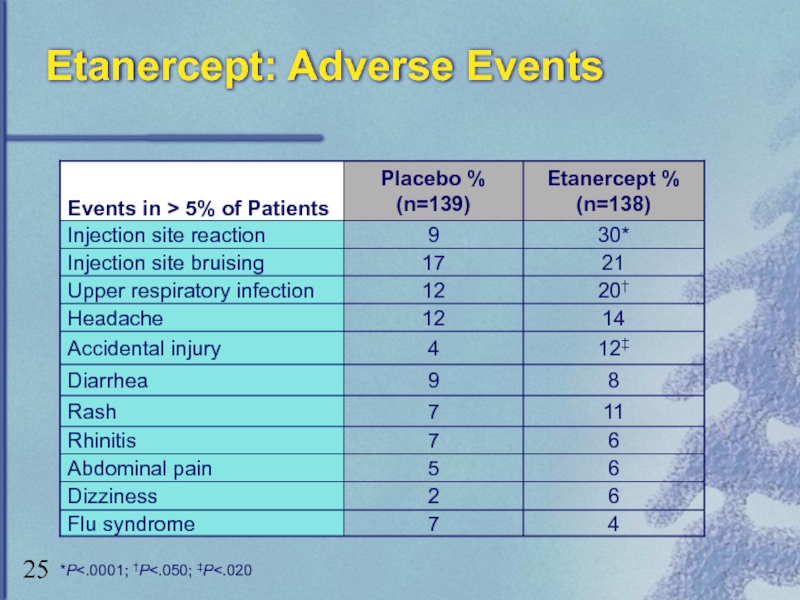
Слайд 26Etanercept: Adverse Events (cont)
Serious infections and sepsis
Mainly in patients with underlying
CNS demyelinating disorders
Causal relationship unclear
Use with caution or avoid use in patients with transverse myelitis, optic neuritis, multiple sclerosis
Pancytopenia
Causal relationship unclear
Use with caution in patients with history of hematologic abnormalities
Autoantibody formation
– Discontinue if lupus-like symptoms are observed
Heart failure
Carefully monitor if prescribed to patients with heart failure
Слайд 27Infliximab for the Treatment of AS: Clinical Trials
Brandt, et al.
≥ 50%
Braun
53% of infliximab-treated patients and 9% placebo-treated patients experienced regression of disease activity of ≥ 50%
Function and quality of life significantly improved with infliximab treatment (P<.0001)
Van den Bosch
Significant improvement with infliximab compared with placebo on patient and physician global assessments of disease activity (P<.001)
Слайд 28Infliximab for the Treatment of AS: Clinical Trials (cont)
Stone, et al.
Improvement
Improvement on MRI scans
Maksymowych, et al.
Significant improvement* on BASDAI; significant mean reduction in BASFI, BASGI, ESR, and CRP at week 14
Efficacy sustained at 1 year
*P<.001, all parameters except CRP, P=.01
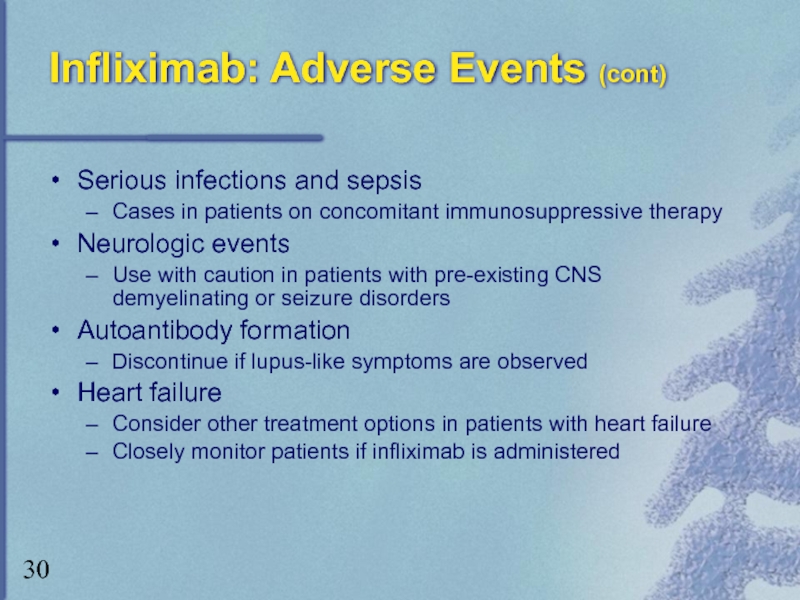
Слайд 30Infliximab: Adverse Events (cont)
Serious infections and sepsis
Cases in patients on concomitant
Neurologic events
Use with caution in patients with pre-existing CNS demyelinating or seizure disorders
Autoantibody formation
Discontinue if lupus-like symptoms are observed
Heart failure
Consider other treatment options in patients with heart failure
Closely monitor patients if infliximab is administered
Слайд 31Anti-TNF Agents: Summary
Anti-TNF agents target underlying inflammatory process
Alter disease progression
Provide symptomatic
Recommended treatment after trial of chronic daily NSAIDs, physical therapy, and regular exercise
Good safety and tolerability profiles
Long-term data needed
Implement treatment guidelines to ensure proper treatment given to appropriate patients
Treatment algorithm presented on next two slides
Слайд 32AS Treatment Algorithm:
Patients with Axial AS
Alternative Options
Pamidronate
Thalidomide
*Only biologic approved for treatment
†Approved in Europe only for treatment of AS
This treatment algorithm contains unlabeled use of infliximab, pamidronate and thalidomide.
Anti-TNF agents
Etanercept 50 mg SC per week as two 25 mg injections in the same day or 3-4 days apart*
Infliximab 5 mg/kg at 0, 2, and 6 weeks and every 6 to 8 weeks thereafter†
Contraindicated in patients with infections, tuberculosis, multiple sclerosis, lupus, malignancy, and pregnancy/lactation
Initiate physical therapy plan with long-
term exercise program to accompany
pharmacologic intervention
Emphasize posture, range of motion, and strengthening
NSAIDs or Selective COX-2 inhibitors
Efficacy and safety comparable between non-selective agents
Selective COX-2 efficacy comparable, better safety profile, higher cost that non-selective NSAIDs
Failure of at least two different NSAIDs/selective COX-2 inhibitors
for minimum of 3 months
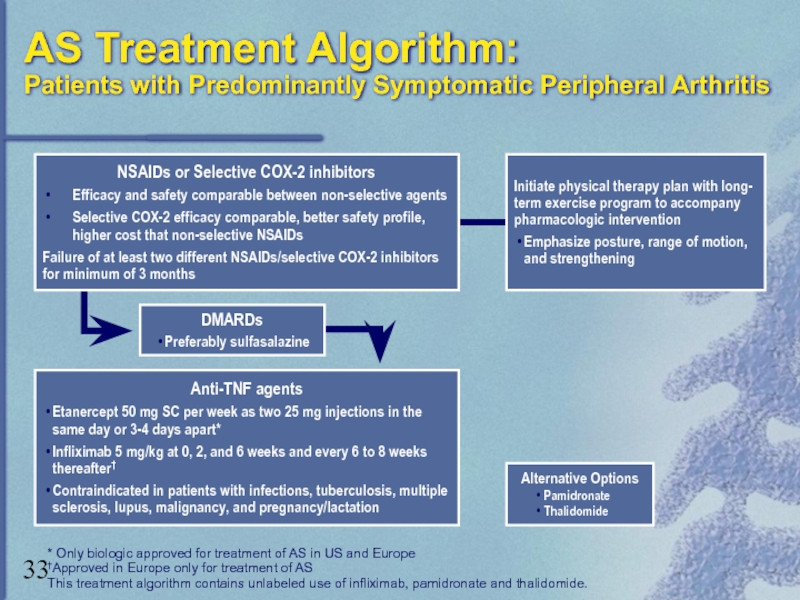
Слайд 33AS Treatment Algorithm:
Patients with Predominantly Symptomatic Peripheral Arthritis
Alternative Options
Pamidronate
Thalidomide
* Only biologic
†Approved in Europe only for treatment of AS
This treatment algorithm contains unlabeled use of infliximab, pamidronate and thalidomide.
Anti-TNF agents
Etanercept 50 mg SC per week as two 25 mg injections in the same day or 3-4 days apart*
Infliximab 5 mg/kg at 0, 2, and 6 weeks and every 6 to 8 weeks thereafter†
Contraindicated in patients with infections, tuberculosis, multiple sclerosis, lupus, malignancy, and pregnancy/lactation
DMARDs
Preferably sulfasalazine
Initiate physical therapy plan with long-
term exercise program to accompany
pharmacologic intervention
Emphasize posture, range of motion, and strengthening
NSAIDs or Selective COX-2 inhibitors
Efficacy and safety comparable between non-selective agents
Selective COX-2 efficacy comparable, better safety profile, higher cost that non-selective NSAIDs
Failure of at least two different NSAIDs/selective COX-2 inhibitors
for minimum of 3 months