- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
What You Need to Know About New Hepatitis C Drugs презентация
Содержание
- 1. What You Need to Know About New Hepatitis C Drugs
- 2. The hepatitis C market is big and
- 3. The 2 Current Leading Treatments Gilead’s Sovaldi
- 4. 3 Treatments awaiting FDA approval 1. Gilead’s
- 5. 3 Treatments awaiting FDA approval 2. AbbVie’s
- 6. 3 Treatments awaiting FDA approval 3. Bristol-Myers
- 7. Fool-Worthy Final Thoughts: Important investor themes Will
- 8. 6 Stock Picks Poised for Incredible Growth
Слайд 2The hepatitis C market is big and global
The most affected regions
are Central and East Asia and North Africa.
An estimated 3 million to 4 million people in the U.S. are have chronic HCV.
The number of people with chronic HCV in the EU totals between 7.3 million and 8.8 million.
Sources: http://www.easl.eu/assets/application/files/bdb06ff135c7ccb_file.pdf
http://www.who.int/mediacentre/factsheets/fs164/en/
An estimated 3 million to 4 million people in the U.S. are have chronic HCV.
The number of people with chronic HCV in the EU totals between 7.3 million and 8.8 million.
Sources: http://www.easl.eu/assets/application/files/bdb06ff135c7ccb_file.pdf
http://www.who.int/mediacentre/factsheets/fs164/en/
130–150 million people globally have chronic hepatitis C infection, with 3 million to 4 million new cases diagnosed annually.
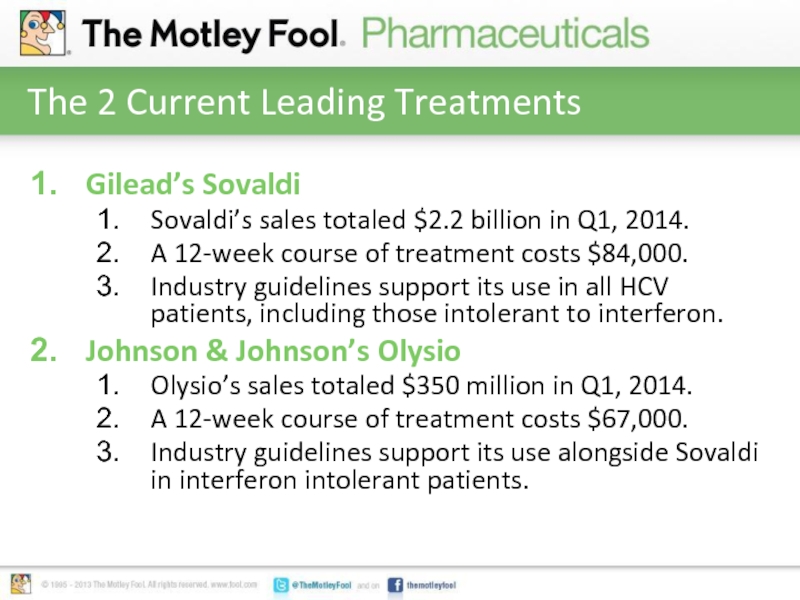
Слайд 3The 2 Current Leading Treatments
Gilead’s Sovaldi
Sovaldi’s sales totaled $2.2 billion in
Q1, 2014.
A 12-week course of treatment costs $84,000.
Industry guidelines support its use in all HCV patients, including those intolerant to interferon.
Johnson & Johnson’s Olysio
Olysio’s sales totaled $350 million in Q1, 2014.
A 12-week course of treatment costs $67,000.
Industry guidelines support its use alongside Sovaldi in interferon intolerant patients.
A 12-week course of treatment costs $84,000.
Industry guidelines support its use in all HCV patients, including those intolerant to interferon.
Johnson & Johnson’s Olysio
Olysio’s sales totaled $350 million in Q1, 2014.
A 12-week course of treatment costs $67,000.
Industry guidelines support its use alongside Sovaldi in interferon intolerant patients.
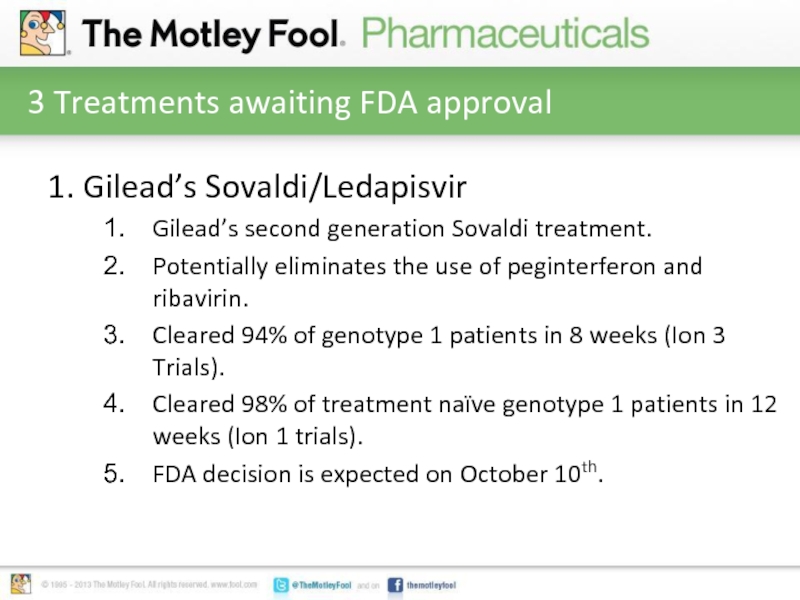
Слайд 43 Treatments awaiting FDA approval
1. Gilead’s Sovaldi/Ledapisvir
Gilead’s second generation Sovaldi treatment.
Potentially
eliminates the use of peginterferon and ribavirin.
Cleared 94% of genotype 1 patients in 8 weeks (Ion 3 Trials).
Cleared 98% of treatment naïve genotype 1 patients in 12 weeks (Ion 1 trials).
FDA decision is expected on October 10th.
Cleared 94% of genotype 1 patients in 8 weeks (Ion 3 Trials).
Cleared 98% of treatment naïve genotype 1 patients in 12 weeks (Ion 1 trials).
FDA decision is expected on October 10th.
Слайд 53 Treatments awaiting FDA approval
2. AbbVie’s 3D
3 drug , 2 pill
combination therapy for use with or without ribavirin.
Received FDA breakthrough status in May 2013.
Cleared 97% of genotype 1a patients when dosed with ribavirin (Pearl IV trials).
Cleared 99% of genotype 1b patients (Pearl III trials).
Filed for FDA approval on April 22nd.
Received FDA breakthrough status in May 2013.
Cleared 97% of genotype 1a patients when dosed with ribavirin (Pearl IV trials).
Cleared 99% of genotype 1b patients (Pearl III trials).
Filed for FDA approval on April 22nd.
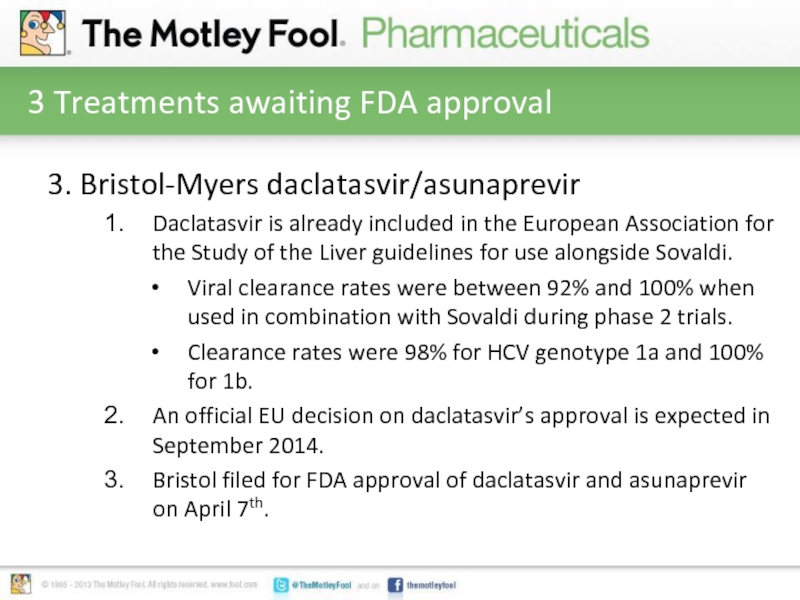
Слайд 63 Treatments awaiting FDA approval
3. Bristol-Myers daclatasvir/asunaprevir
Daclatasvir is already included in
the European Association for the Study of the Liver guidelines for use alongside Sovaldi.
Viral clearance rates were between 92% and 100% when used in combination with Sovaldi during phase 2 trials.
Clearance rates were 98% for HCV genotype 1a and 100% for 1b.
An official EU decision on daclatasvir’s approval is expected in September 2014.
Bristol filed for FDA approval of daclatasvir and asunaprevir on April 7th.
Viral clearance rates were between 92% and 100% when used in combination with Sovaldi during phase 2 trials.
Clearance rates were 98% for HCV genotype 1a and 100% for 1b.
An official EU decision on daclatasvir’s approval is expected in September 2014.
Bristol filed for FDA approval of daclatasvir and asunaprevir on April 7th.
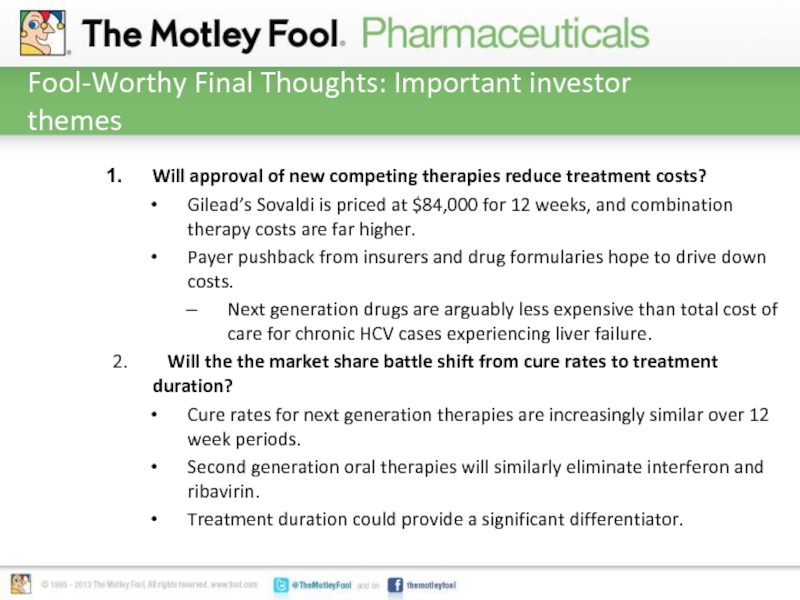
Слайд 7Fool-Worthy Final Thoughts: Important investor themes
Will approval of new competing therapies
reduce treatment costs?
Gilead’s Sovaldi is priced at $84,000 for 12 weeks, and combination therapy costs are far higher.
Payer pushback from insurers and drug formularies hope to drive down costs.
Next generation drugs are arguably less expensive than total cost of care for chronic HCV cases experiencing liver failure.
2. Will the the market share battle shift from cure rates to treatment duration?
Cure rates for next generation therapies are increasingly similar over 12 week periods.
Second generation oral therapies will similarly eliminate interferon and ribavirin.
Treatment duration could provide a significant differentiator.
Gilead’s Sovaldi is priced at $84,000 for 12 weeks, and combination therapy costs are far higher.
Payer pushback from insurers and drug formularies hope to drive down costs.
Next generation drugs are arguably less expensive than total cost of care for chronic HCV cases experiencing liver failure.
2. Will the the market share battle shift from cure rates to treatment duration?
Cure rates for next generation therapies are increasingly similar over 12 week periods.
Second generation oral therapies will similarly eliminate interferon and ribavirin.
Treatment duration could provide a significant differentiator.