PharmaSUG 2014
Annual Conference
- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Accenture Accelerated R&DCDISC Electronic Submission to FDAKevin LeeClinical Data Strategies, Senior Consultant презентация
Содержание
- 1. Accenture Accelerated R&DCDISC Electronic Submission to FDAKevin LeeClinical Data Strategies, Senior Consultant
- 2. Disclaimer Any views or opinions presented in
- 3. Why? © 2014 Accenture All Rights Reserved.
- 4. Agenda © 2014 Accenture All Rights Reserved.
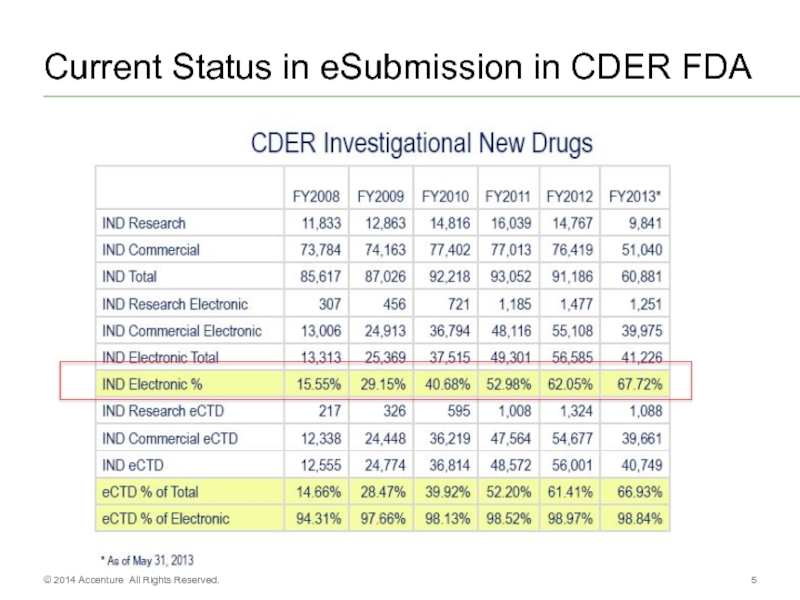
- 5. Current Status in eSubmission in CDER FDA © 2014 Accenture All Rights Reserved.
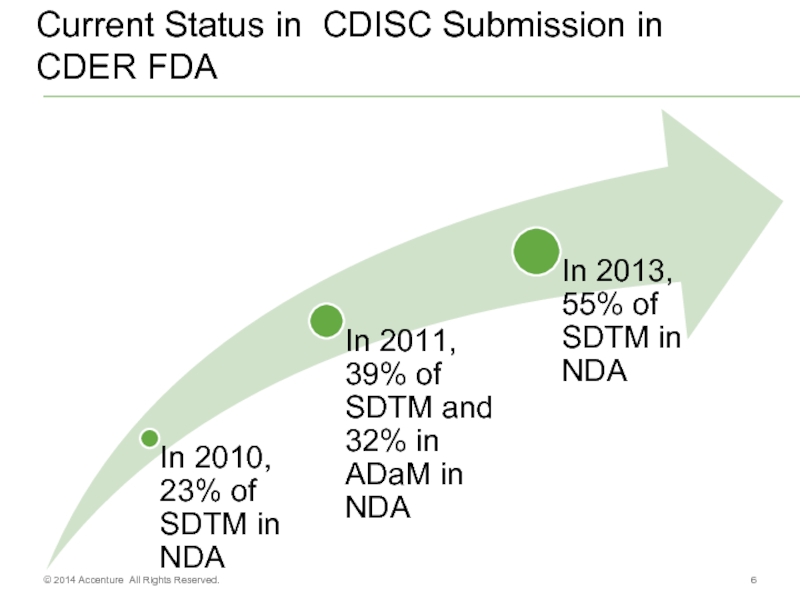
- 6. Current Status in CDISC Submission in CDER FDA © 2014 Accenture All Rights Reserved.
- 7. Section 745A(a) of the Federal Food, Drug,
- 8. New FDA Draft Guidance on CDISC eSubmission © 2014 Accenture All Rights Reserved.
- 9. How to prepare CDISC eSubmission © 2014
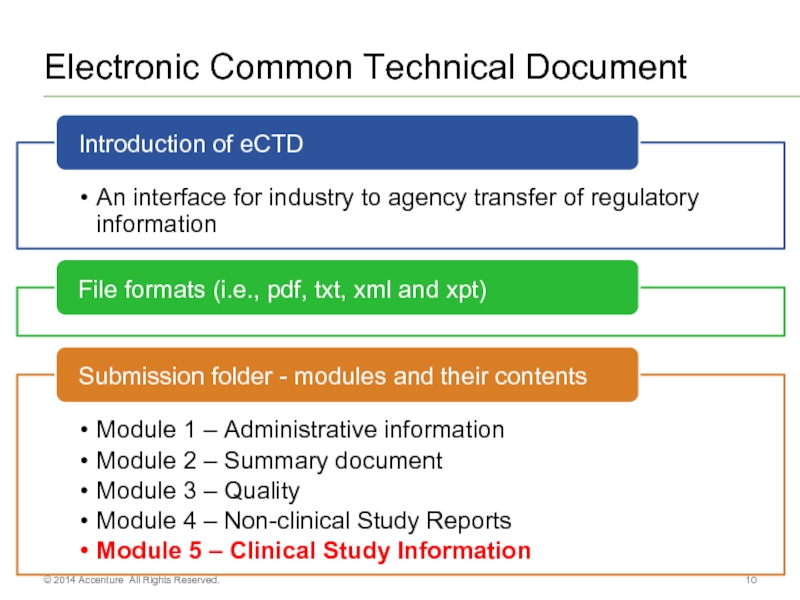
- 10. Electronic Common Technical Document © 2014 Accenture All Rights Reserved.
- 11. Data Standards Catalog © 2014 Accenture All
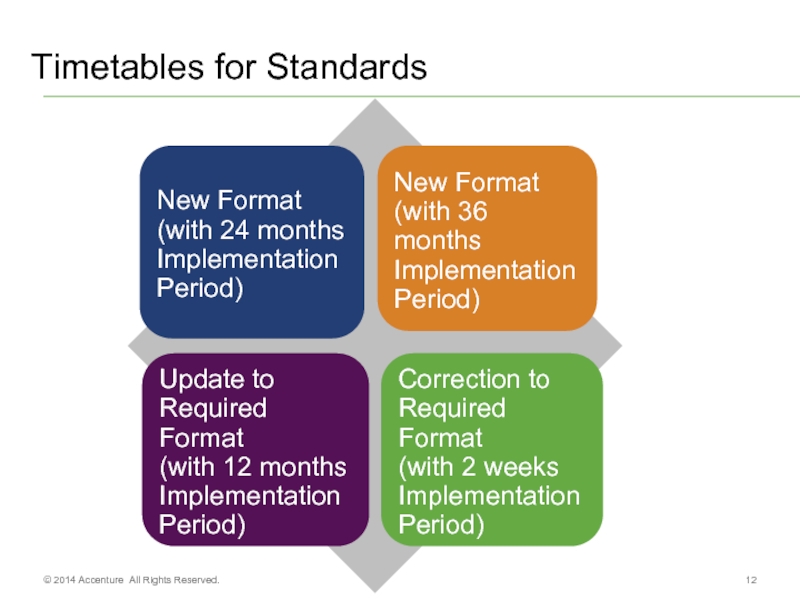
- 12. Timetables for Standards © 2014 Accenture All Rights Reserved.
- 13. Timetable Example (Update – 12 months Implementation)
- 14. Study Data Technical Conformance Guide © 2014 Accenture All Rights Reserved.
- 15. What to prepare for CDISC eSubmission ©
- 16. CDISC Clinical Trial Process © 2014 Accenture
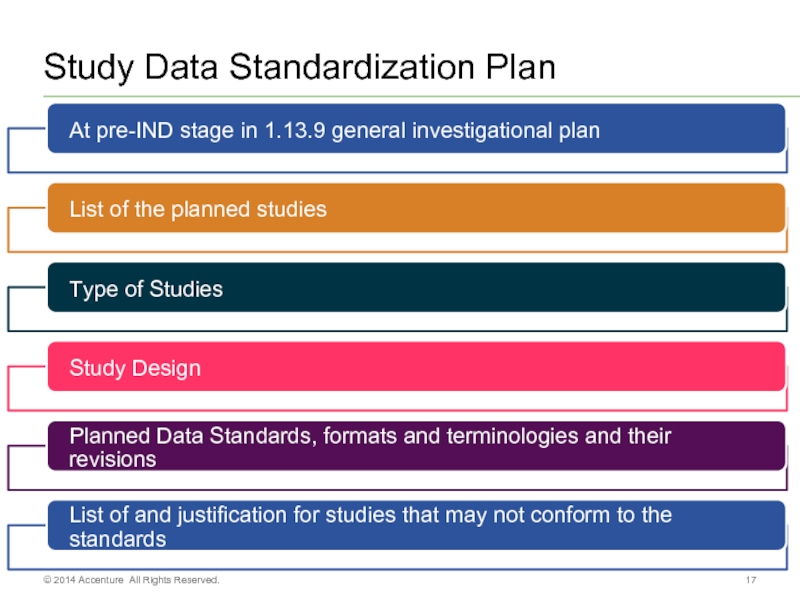
- 17. Study Data Standardization Plan © 2014 Accenture All Rights Reserved.
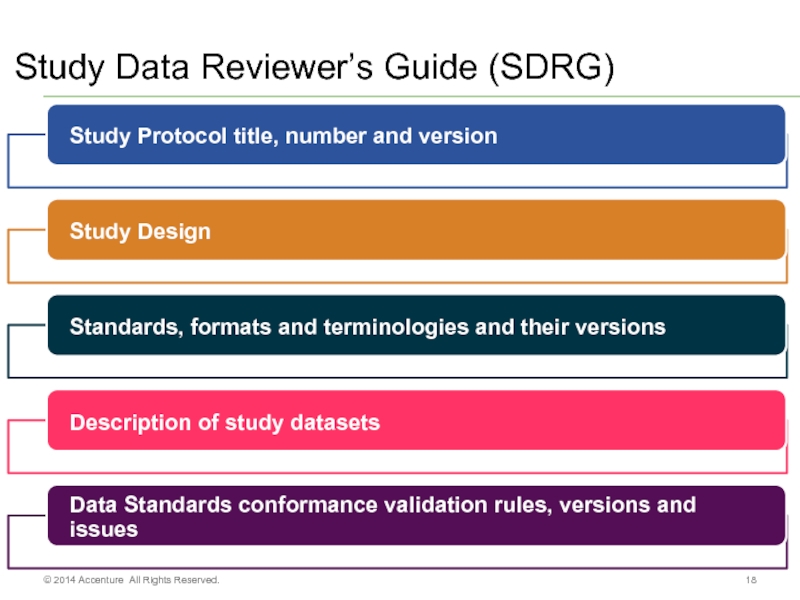
- 18. Study Data Reviewer’s Guide (SDRG) © 2014 Accenture All Rights Reserved.
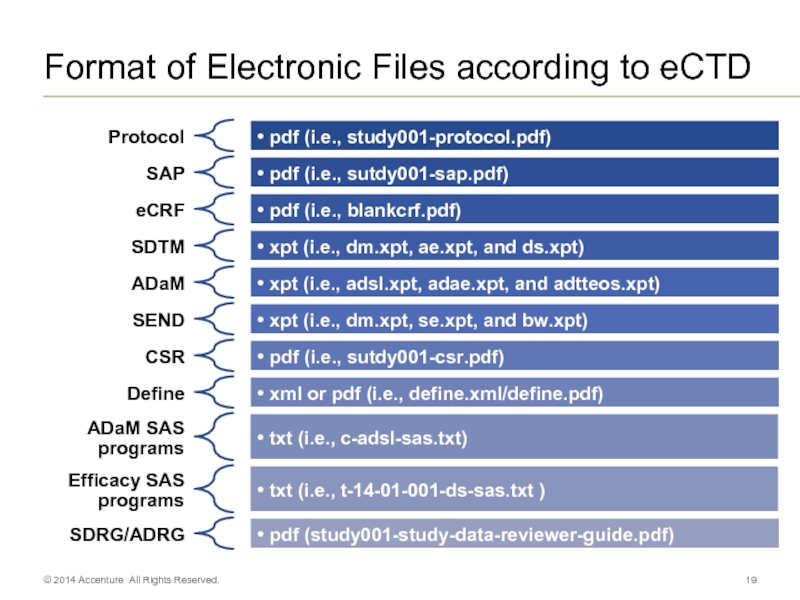
- 19. Format of Electronic Files according to eCTD © 2014 Accenture All Rights Reserved.
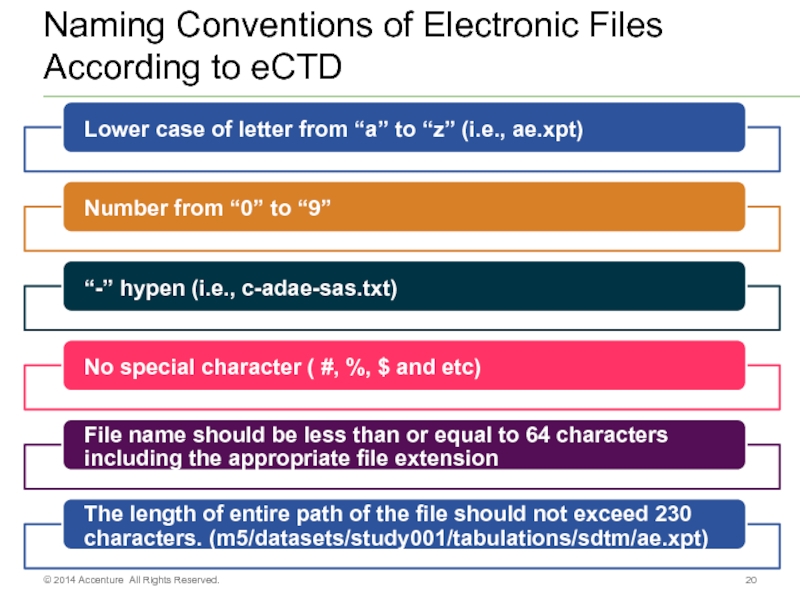
- 20. Naming Conventions of Electronic Files According to eCTD © 2014 Accenture All Rights Reserved.
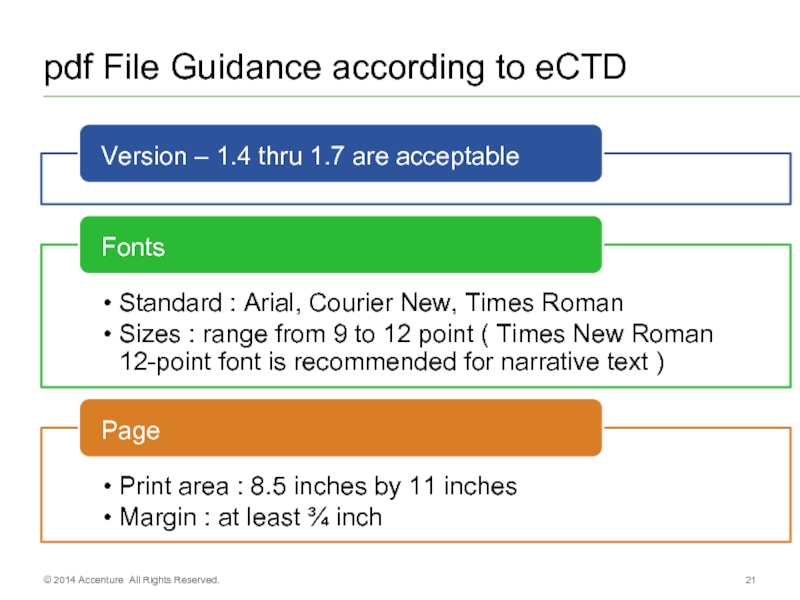
- 21. pdf File Guidance according to eCTD © 2014 Accenture All Rights Reserved.
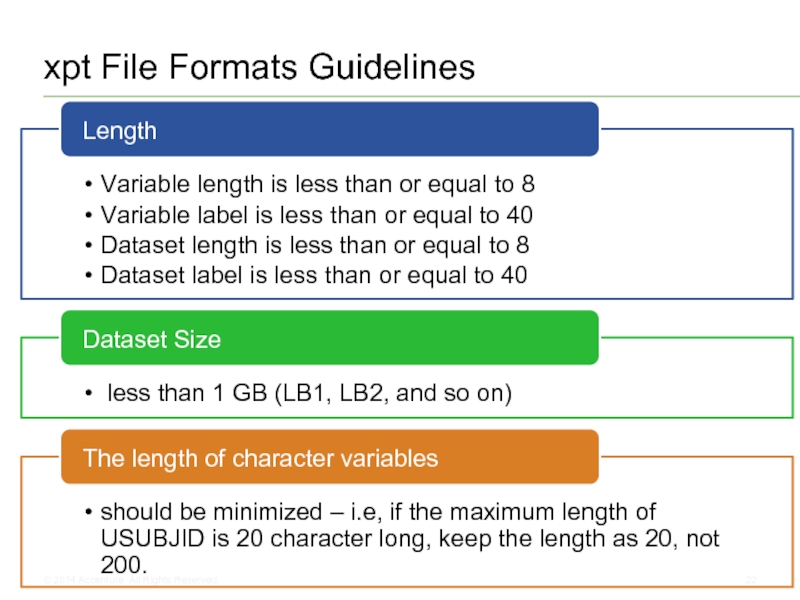
- 22. xpt File Formats Guidelines © 2014 Accenture All Rights Reserved.
- 23. Reports in module 5.3.1.1 of eCTD ©
- 24. CDISC Datasets in module 5.datasets © 2014
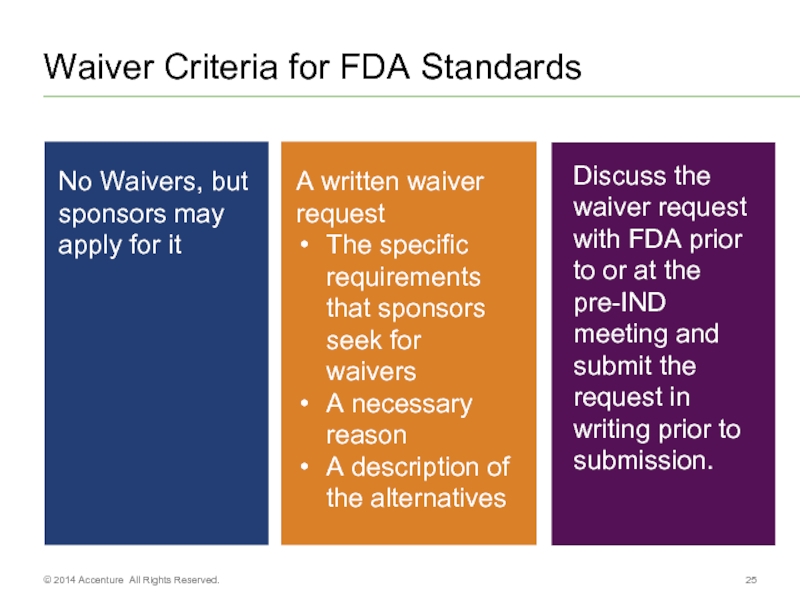
- 25. Waiver Criteria for FDA Standards © 2014 Accenture All Rights Reserved.
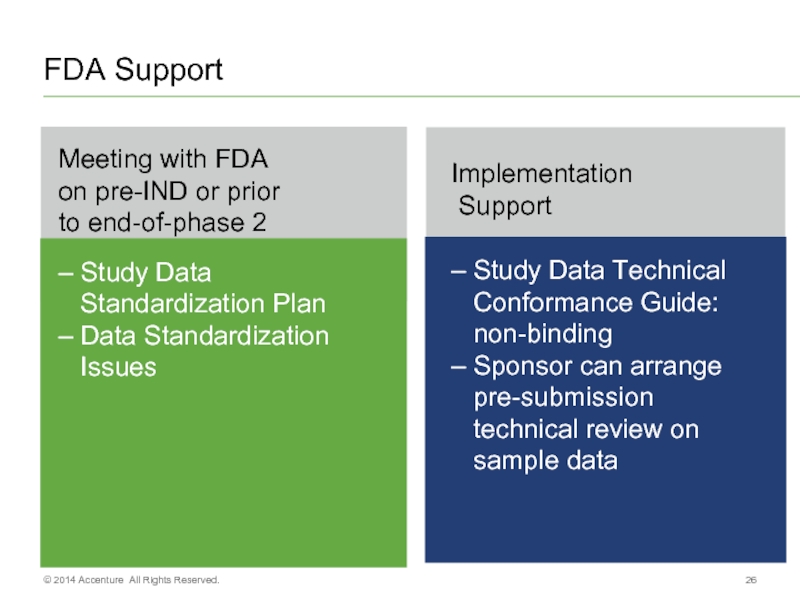
- 26. FDA Support © 2014 Accenture All Rights Reserved.
- 27. Conclusion © 2014 Accenture All Rights Reserved.
- 28. Contact Information © 2014 Accenture All Rights
- 29. Questions and Discussion © 2014 Accenture All Rights Reserved.
Слайд 1Accenture Accelerated R&D CDISC Electronic Submission to FDA Kevin Lee Clinical Data Strategies, Senior
Слайд 2Disclaimer
Any views or opinions presented in this presentation are solely those
© 2014 Accenture All Rights Reserved.
Слайд 4Agenda
© 2014 Accenture All Rights Reserved.
Why do we care CDISC electronic
How can we prepare CDISC electronic submission?
What do we prepare CDISC electronic submission?
Conclusion
Questions & Answers
Слайд 7Section 745A(a) of the Federal Food, Drug, and Cosmetic Act (FD&C
© 2014 Accenture All Rights Reserved.
Enhanced by Food and Drug Administration Safety and Innovation Act (FDASIA) on July 9, 2012.
Requires that submissions be submitted in electronic format.
Слайд 9How to prepare CDISC eSubmission
© 2014 Accenture All Rights Reserved.
eCTD(Electronic Common
Data Standard Catalog
CDISC Standards
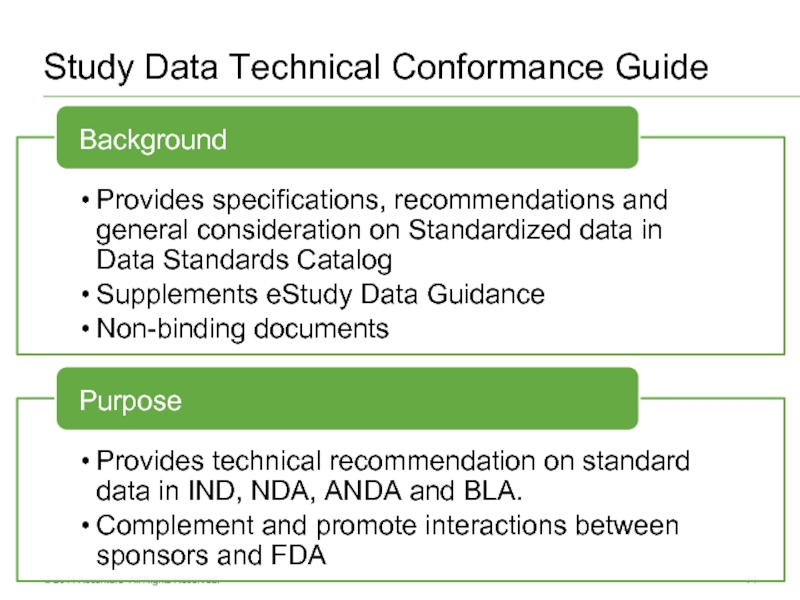
Study Data Technical Conformance Guide
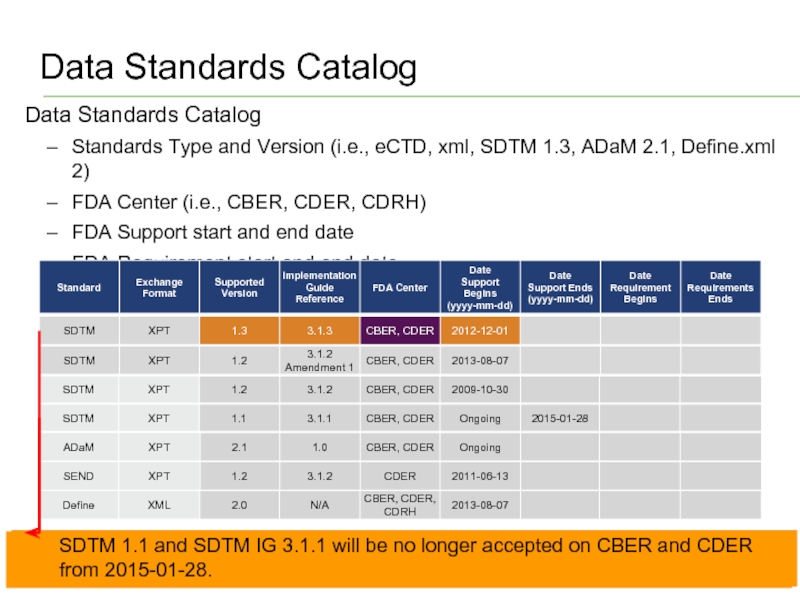
Слайд 11Data Standards Catalog
© 2014 Accenture All Rights Reserved.
Data Standards Catalog
Standards Type
FDA Center (i.e., CBER, CDER, CDRH)
FDA Support start and end date
FDA Requirement start and end date
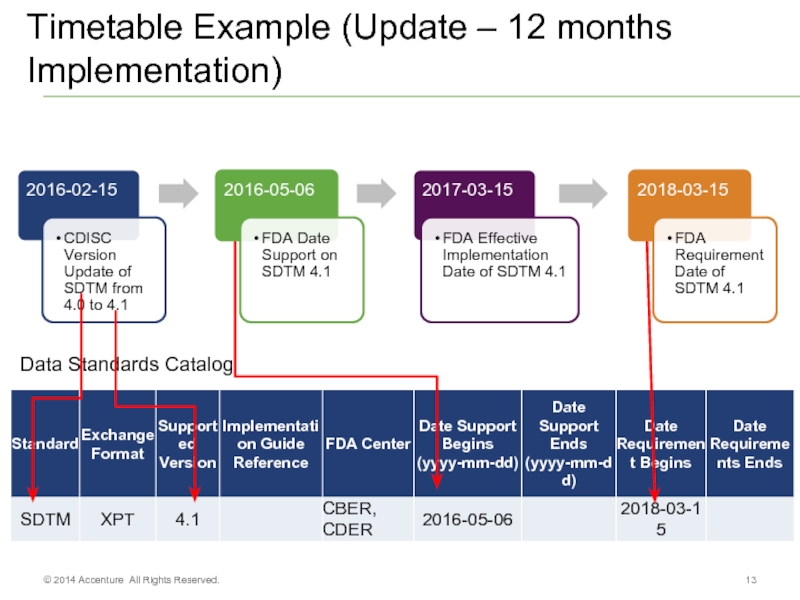
SDTM 1.3 and SDTM IG 3.1.3 is accepted on CBER and CDER from 2012-12-01 on xpt format.
SDTM 1.1 and SDTM IG 3.1.1 will be no longer accepted on CBER and CDER from 2015-01-28.
Слайд 13Timetable Example (Update – 12 months Implementation)
© 2014 Accenture All Rights
Data Standards Catalog
Слайд 15What to prepare for CDISC eSubmission
© 2014 Accenture All Rights Reserved.
CDISC
Its electronic formats according to eCTD
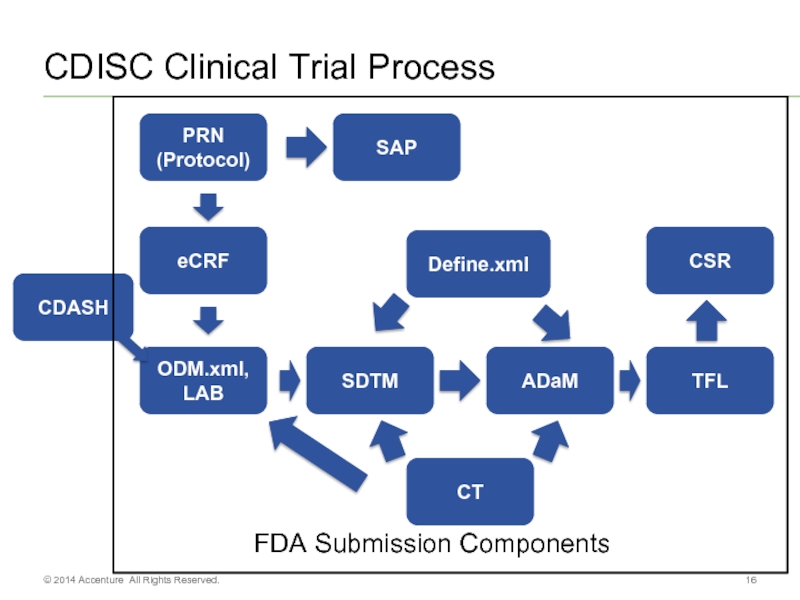
Слайд 16CDISC Clinical Trial Process
© 2014 Accenture All Rights Reserved.
PRN (Protocol)
eCRF
ODM.xml, LAB
SDTM
TFL
ADaM
SAP
CDASH
CSR
CT
Define.xml
FDA
Слайд 20Naming Conventions of Electronic Files According to eCTD
© 2014 Accenture All
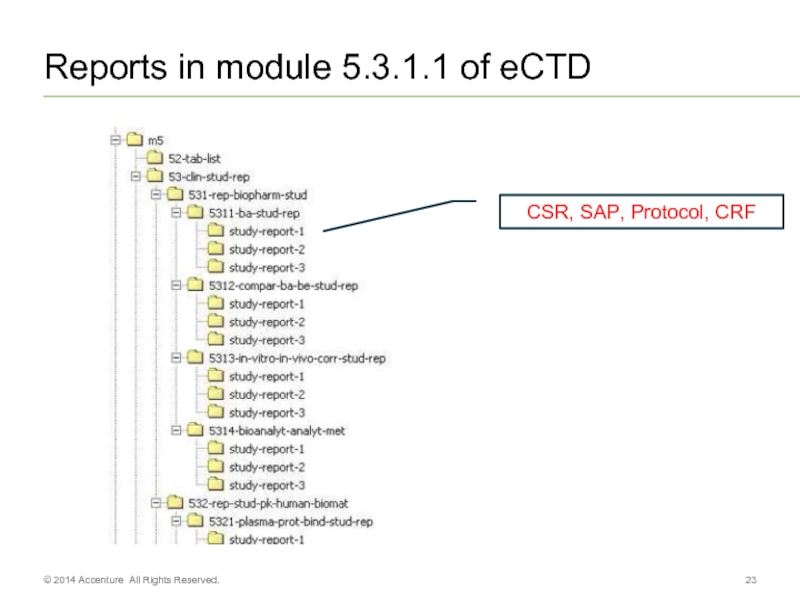
Слайд 23Reports in module 5.3.1.1 of eCTD
© 2014 Accenture All Rights Reserved.
CSR,
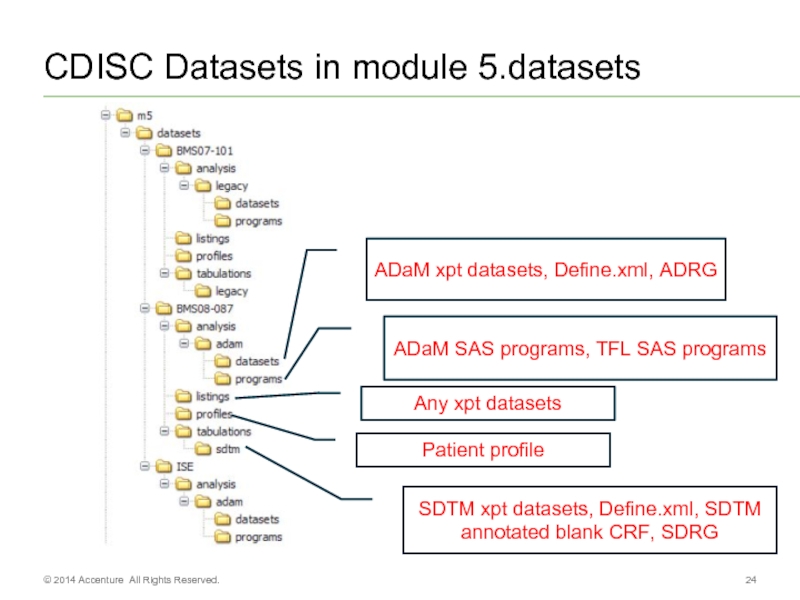
Слайд 24CDISC Datasets in module 5.datasets
© 2014 Accenture All Rights Reserved.
ADaM xpt
ADaM SAS programs, TFL SAS programs
SDTM xpt datasets, Define.xml, SDTM annotated blank CRF, SDRG
Any xpt datasets
Patient profile
Слайд 27Conclusion
© 2014 Accenture All Rights Reserved.
Before : “should” in FDA documents
After : “should” in Guidance for Industry in Electronic Submission means required.
It is better to start with the end in minds.
Слайд 28Contact Information
© 2014 Accenture All Rights Reserved.
Email address : kevin.s.lee@accenture.com
Linkedin Profile
Tweet : @HelloKevinLee
Slide share : http://www.slideshare.net/KevinLee56
Blogs : HiKevinLee.tumbrl.com