- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Solutions. Acid–base equilibrium in biological systems презентация
Содержание
- 1. Solutions. Acid–base equilibrium in biological systems
- 2. Plan 0. Solutions and their
- 19. Theory of electrolytic dissociation (Arrhenius’
- 20. The theory of electrolytic dissociation
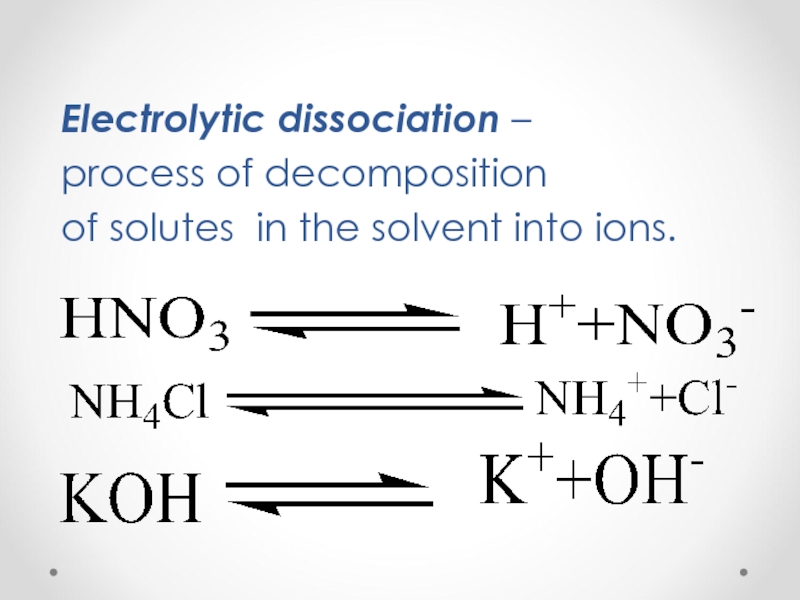
- 22. Electrolytic dissociation – process of
- 23. 1) Substances dissociating in solutions or
- 24. Dissociation of bases, acides and salts in water solutions
- 25. Acides are compounds dissociating in aqueous solutions
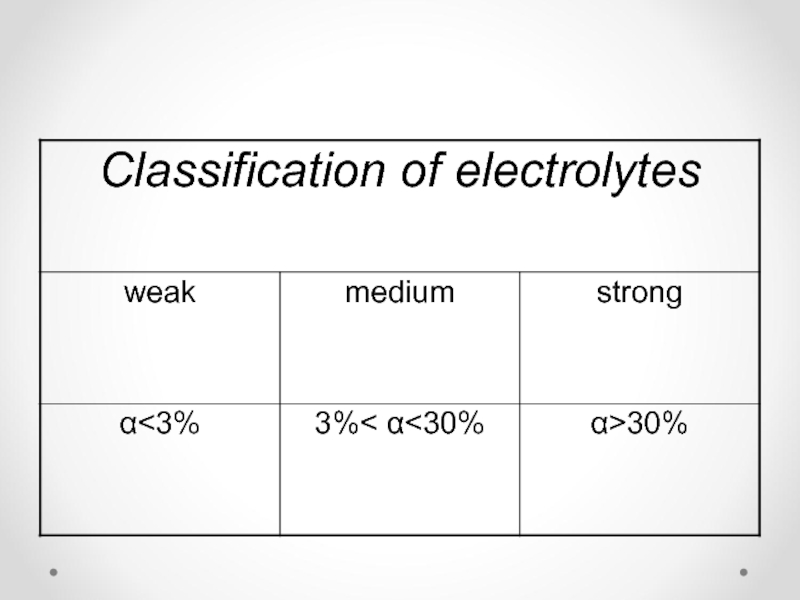
- 26. Strong and weak electrolytes
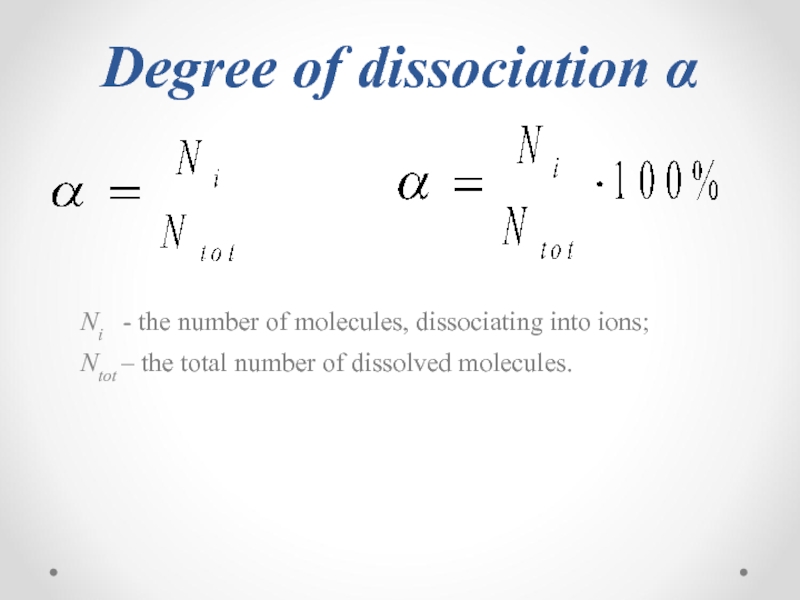
- 27. Degree of dissociation α
- 29. Strong electrolytes Majority of salts. Some
- 30. Weak electrolytes Majority of acids and bases (H2S, H2CO3, Al(OH)3, NH4OH).
- 31. The dissociation of weak electrolytes is a
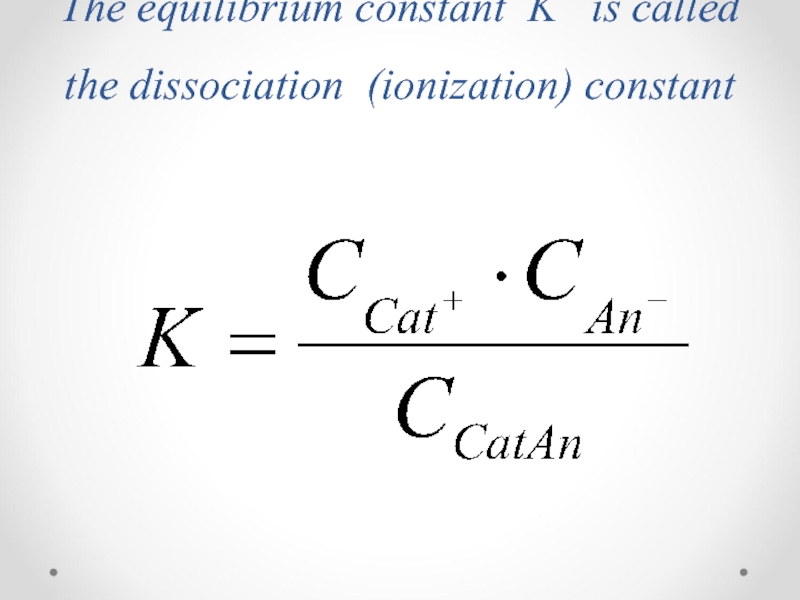
- 32. The equilibrium constant K is called the dissociation (ionization) constant
- 33. Ostwald dilution law Because in solutions of
- 34. Acidity and basicity constants The dissociation constants
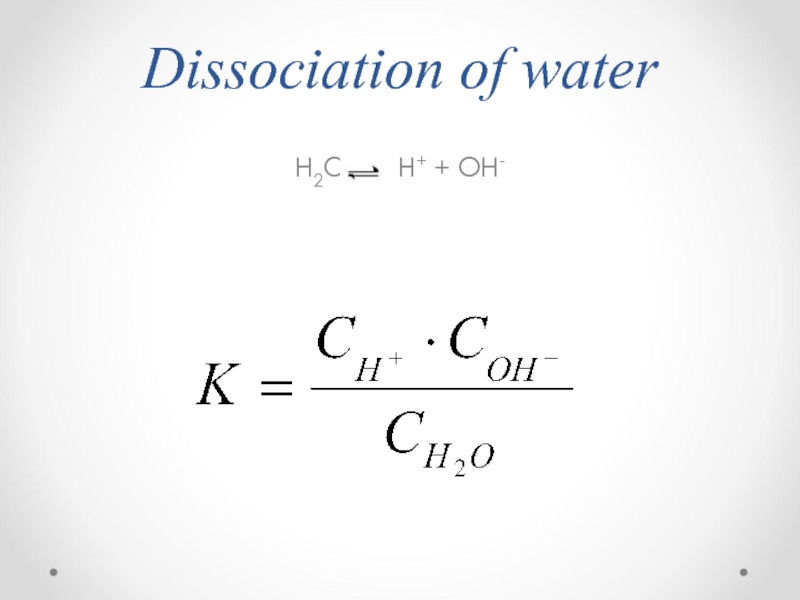
- 36. Dissociation of water H2O H+ + OH-
- 37. Kw is constant, ion product of water.
- 38. Hydrogen ion exponent pH= -lg [H+]
- 39. pH Measurement indicators pH - meters
- 41. Protolytic theory Danish physicist and chemist Johannes
- 42. Base - a substance (particle) that
- 43. Salt - the reaction product of acid
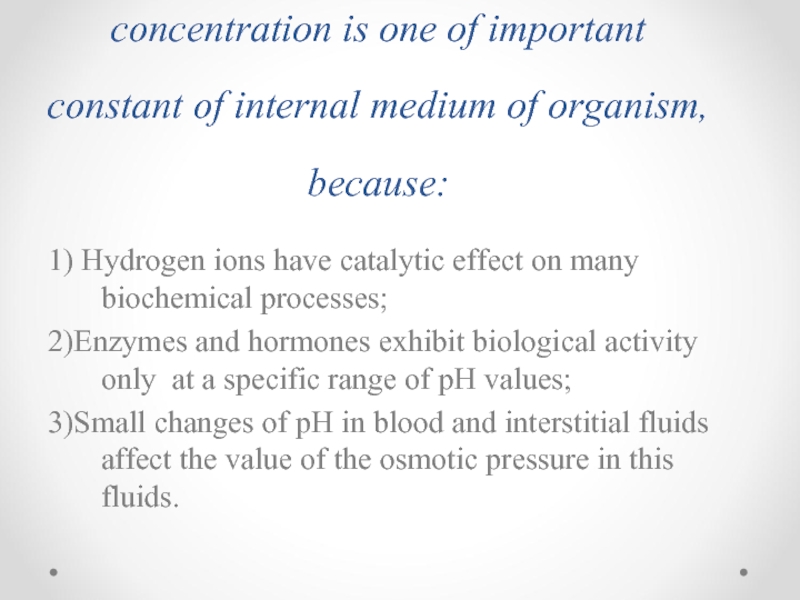
- 44. The homeostasis. The importancy of pH maintenance
- 45. The constancy of hydrogen ions concentration is
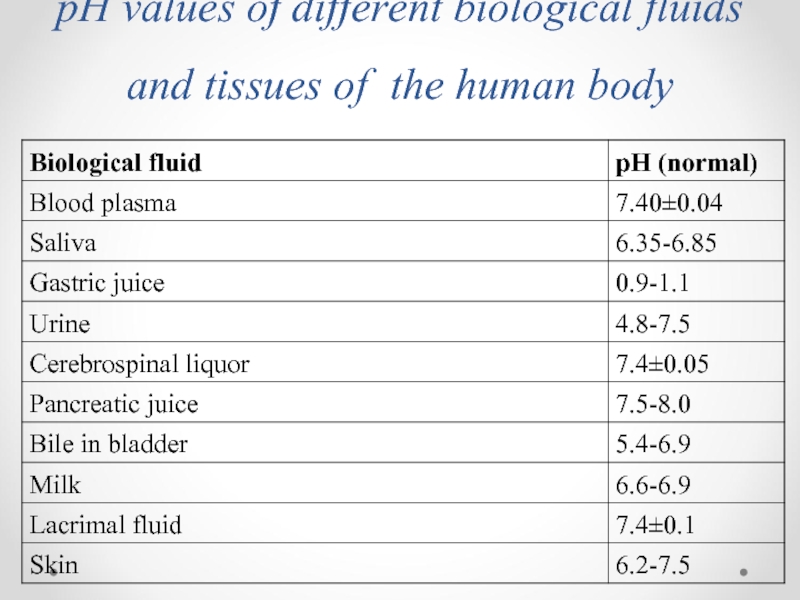
- 46. pH values of different biological fluids and tissues of the human body
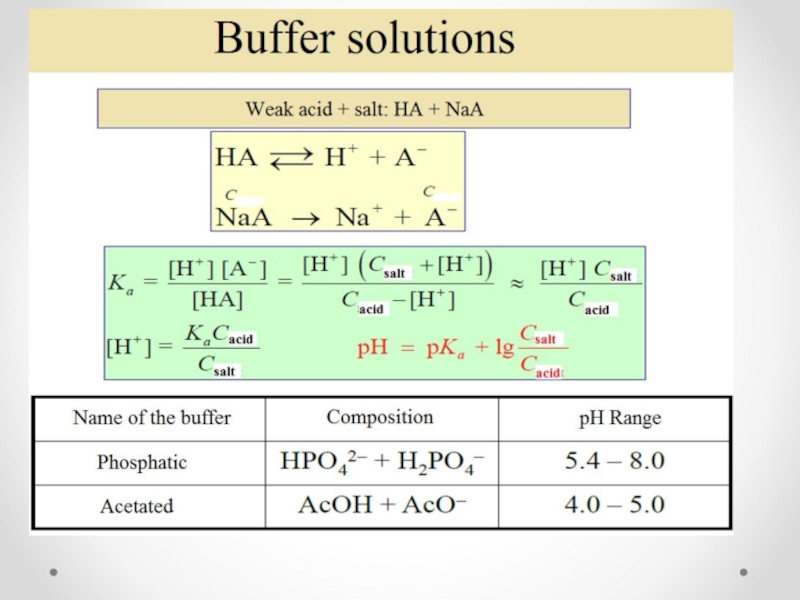
- 47. The concept of buffer solutions Buffer solutions
- 49. The resistive action is the result of
- 50. Henderson-Hasselbah equation
- 51. Buffer capacity Buffer capacity (B) - the
- 52. Buffer capacity Buffer capacity is maximal at
- 53. The relative contribution% buffer
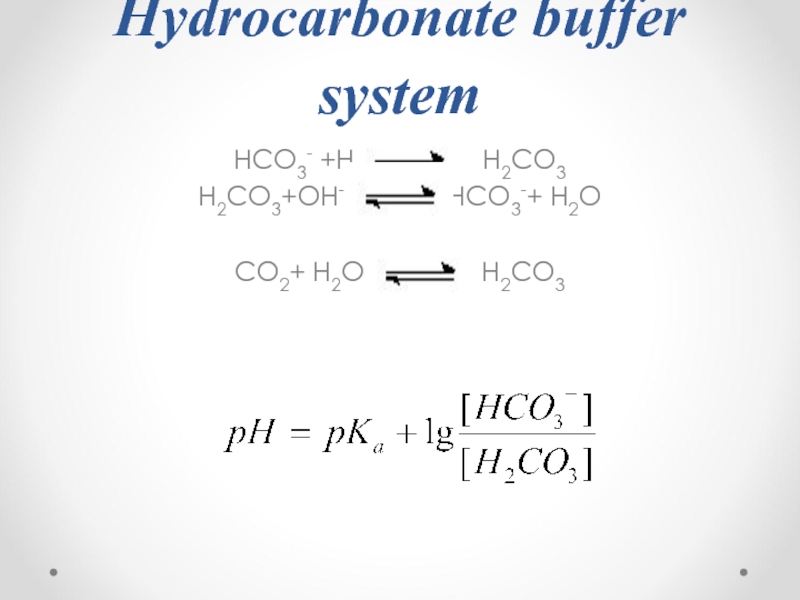
- 54. Hydrocarbonate buffer system HCO3- +H+
- 55. pKa1(H2CO3)=6.1 pH of a blood plasma = 7.4
- 56. Alkaline reserve HCO3-+ H+
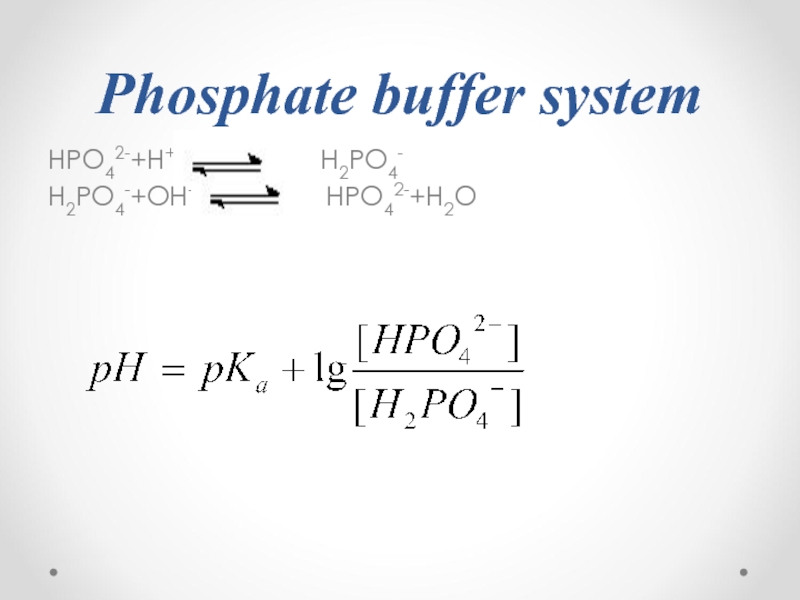
- 57. Phosphate buffer system HPO42-+H+
- 58. The mechanism of action of phosphate
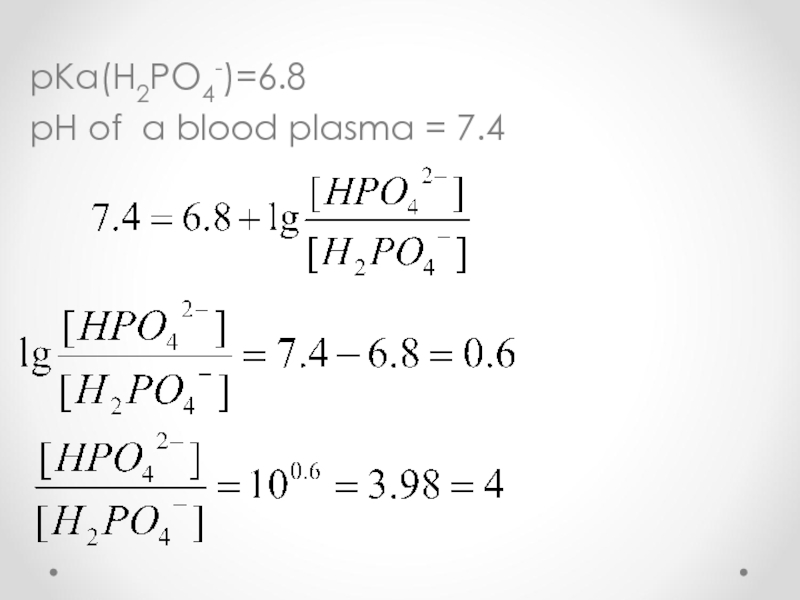
- 59. pKa(H2PO4-)=6.8 pH of a blood
- 60. Protein buffer systems The plasma
- 61. PROTEIN acid-base buffer system
- 62. Hemoglobin buffer system
- 64. Hemoglobin acid-base buffer system BLOOD
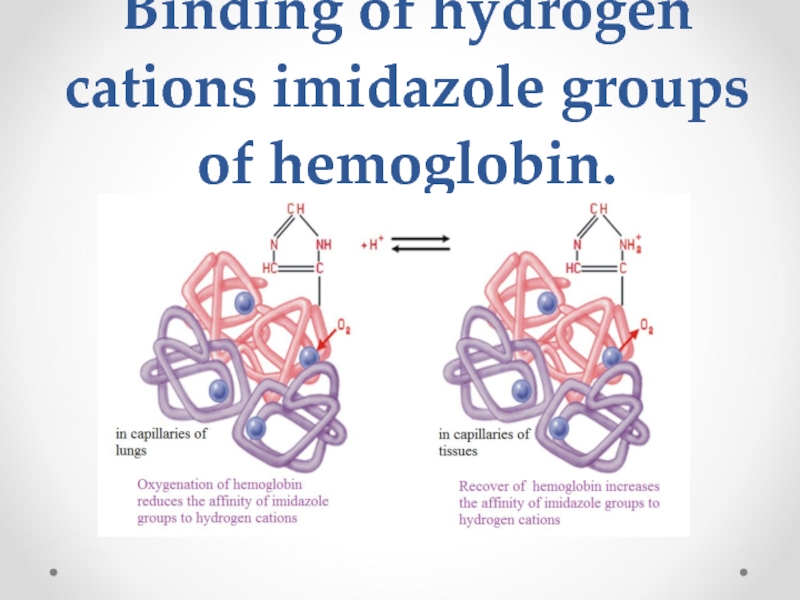
- 65. Binding of hydrogen cations imidazole groups of hemoglobin.
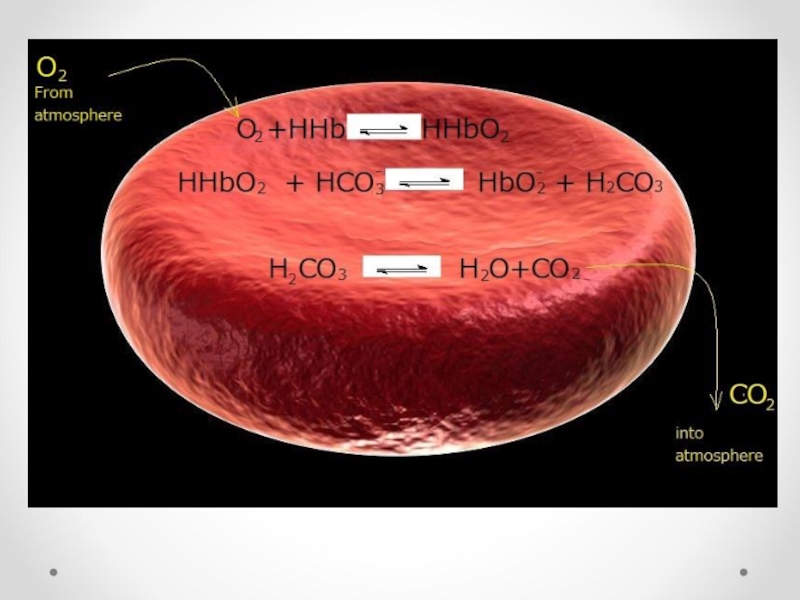
- 66. Hemoglobin buffer system HHb + O2
- 67. a) the hemoglobin buffer system:
- 68. In erythrocytes: HHbO2
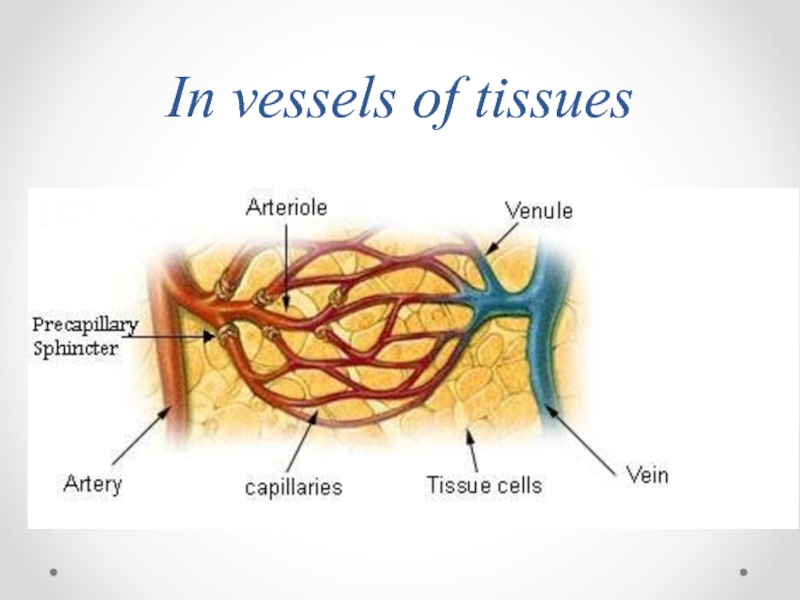
- 69. In vessels of tissues
- 71. In vessels of tissues CO2+ H2O
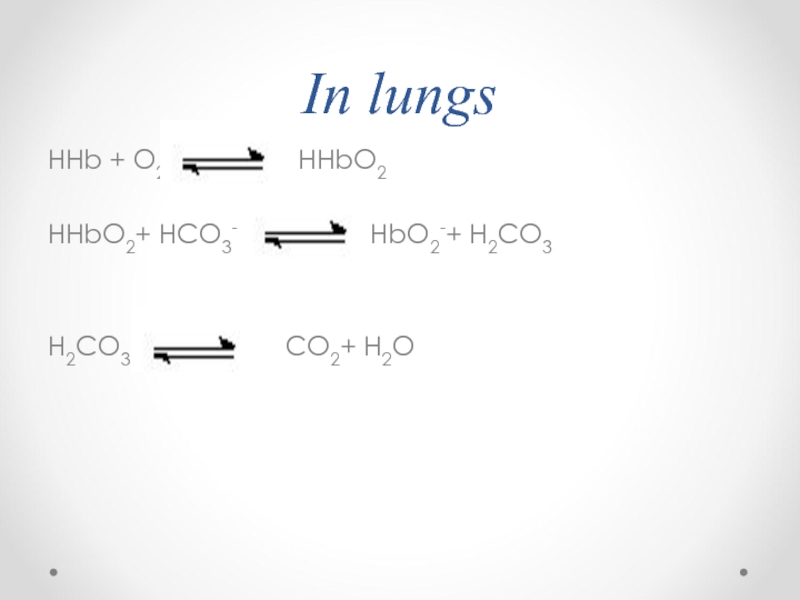
- 72. In lungs
- 74. In lungs HHb + O2
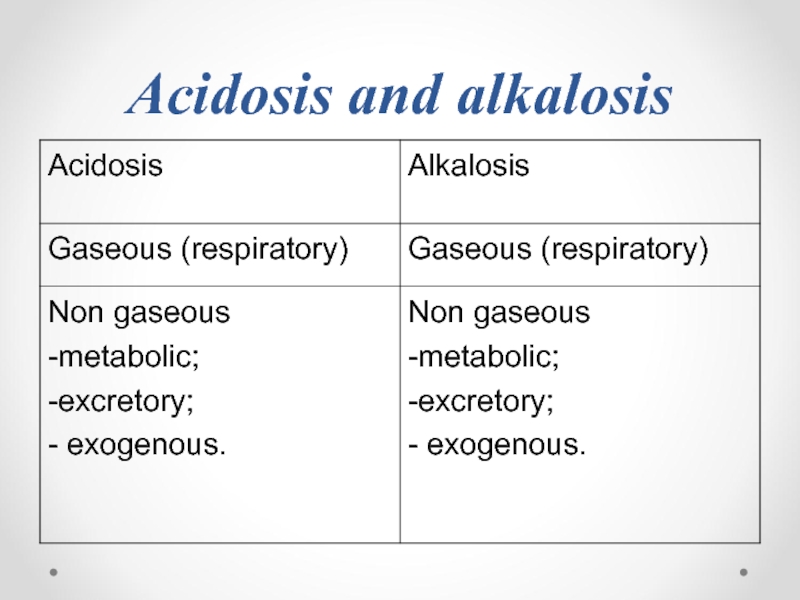
- 75. Acidosis and alkalosis
- 76. Literature 1. Medical Chemistry : textbook /
Слайд 2Plan
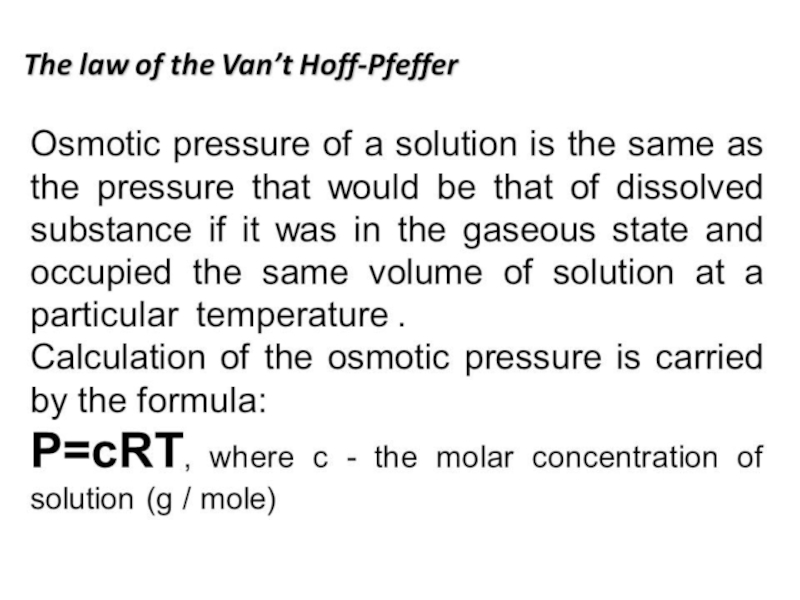
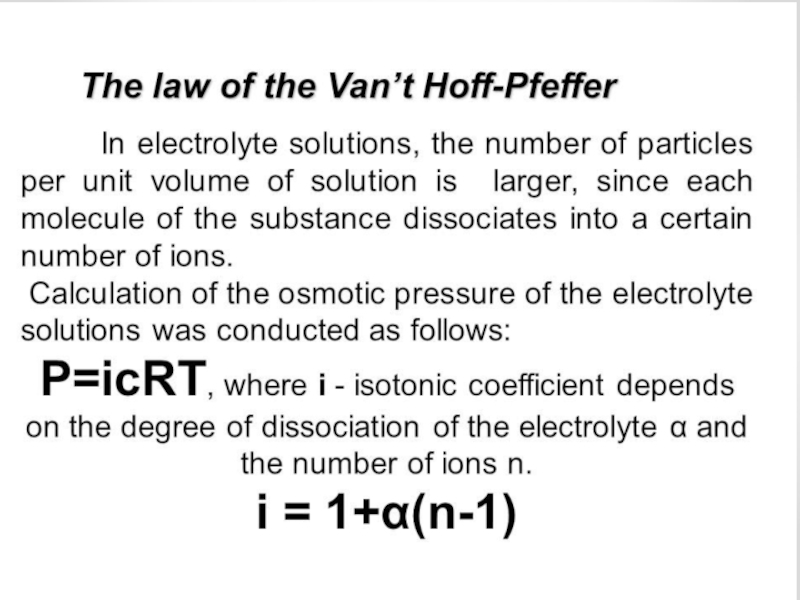
0. Solutions and their colligative properties
1. The theory of
2. Protolytic theory.
3. Dissociation of water. Hydrogen ion exponent.
The homeostasis.
4. The importancy of pH maintenance in human body. 5. The concept of buffer solutions.
6. Hydrocarbonate buffer system
7. Phosphate buffer system
8. Protein buffer systems
9. Hemoglobin buffer system
10. Acidosis and alkalosis. Treatment of acidosis and alkalosis.
Слайд 19
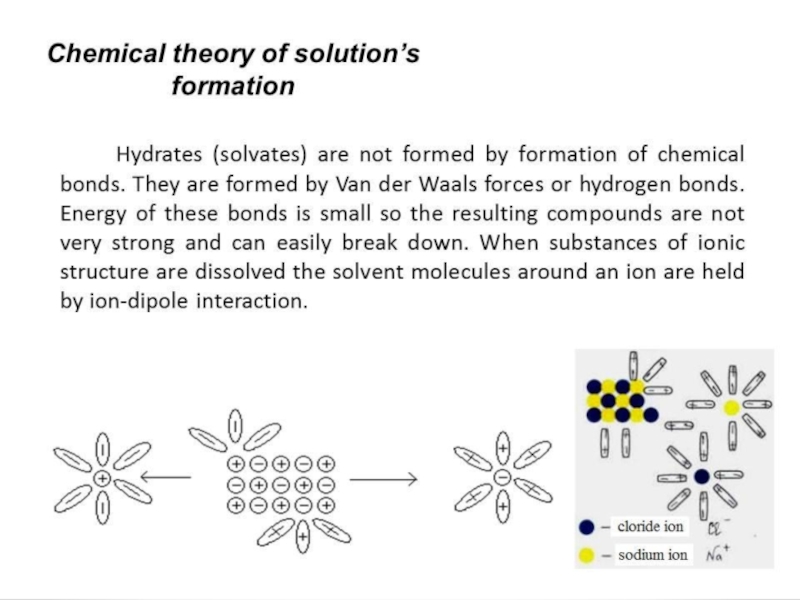
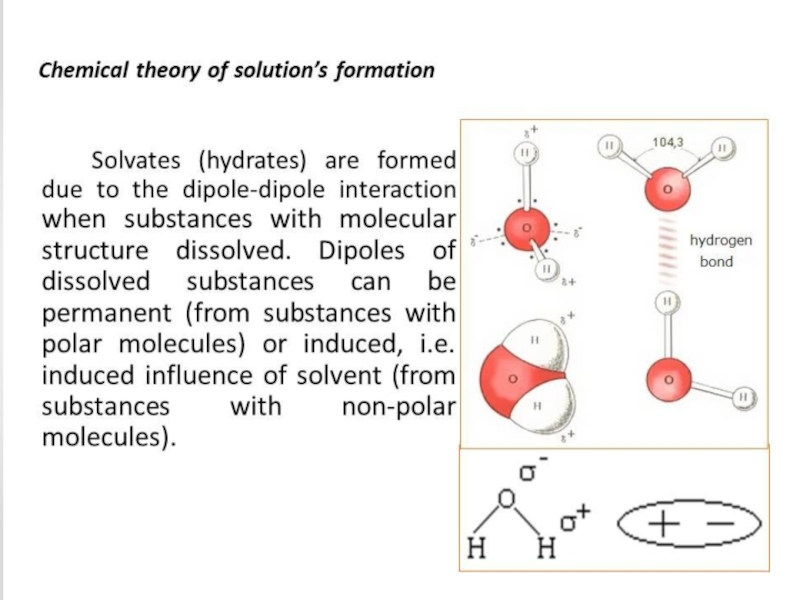
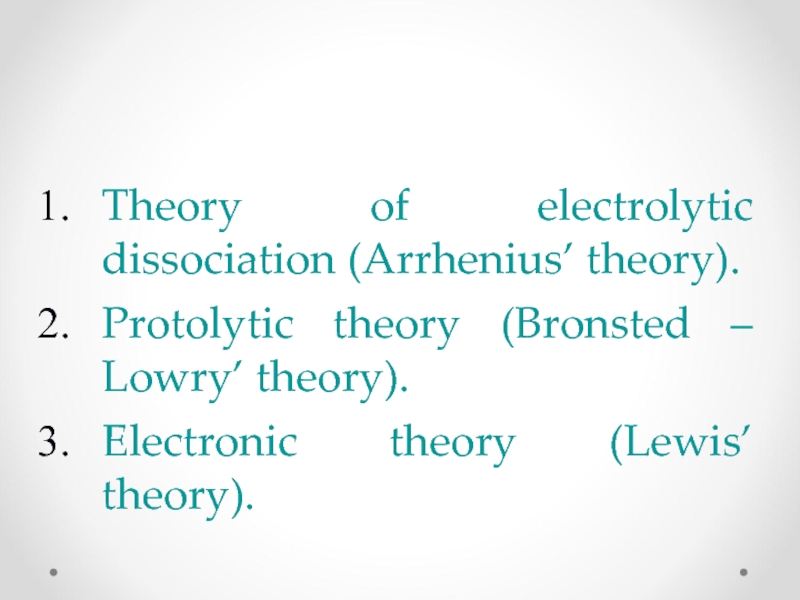
Theory of electrolytic dissociation (Arrhenius’ theory).
Protolytic theory (Bronsted – Lowry’ theory).
Electronic
Слайд 23
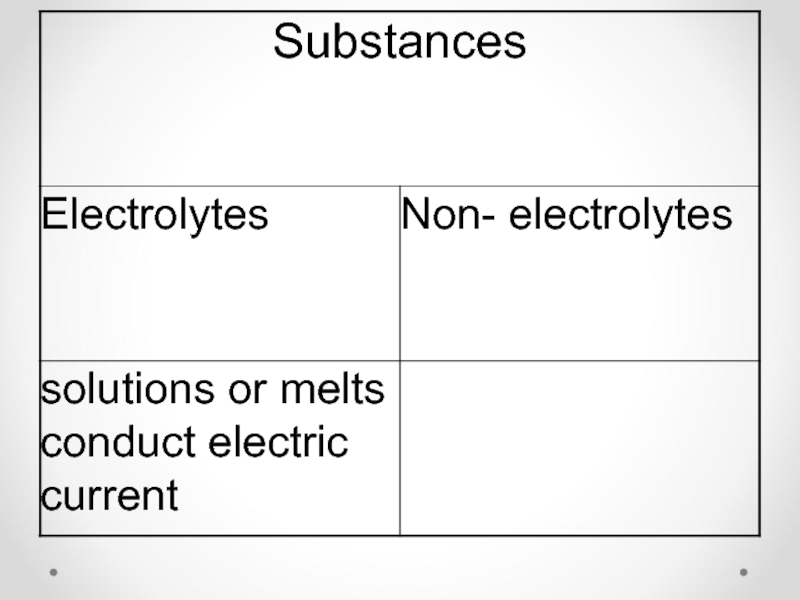
1) Substances dissociating in solutions or melts into positively charged Cat+(cations)
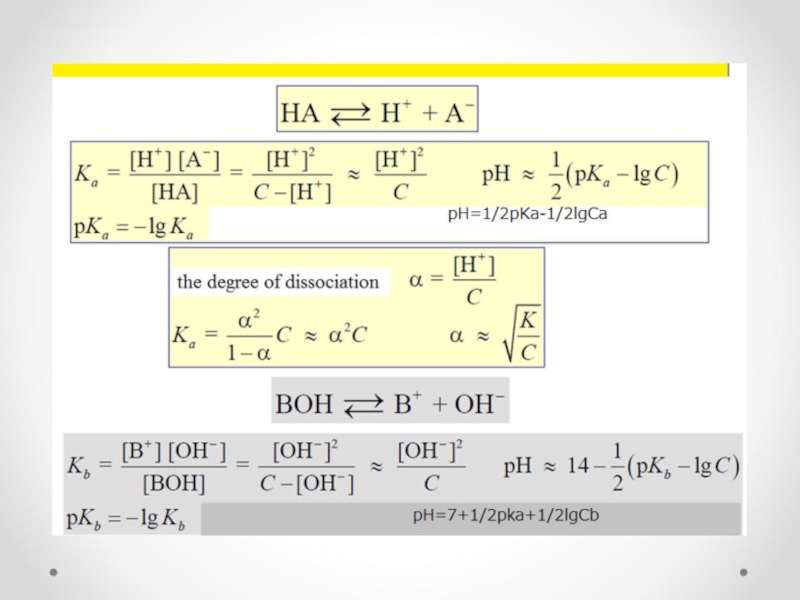
Слайд 25Acides are compounds dissociating in aqueous solutions with the formation of
Слайд 27Degree of dissociation α
Ni - the number of molecules,
Ntot – the total number of dissolved molecules.
Слайд 29Strong electrolytes
Majority of salts.
Some acids (HCl, HBr, HI, HNO3, HClO4,
Alkalis (LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH)2 , Sr(OH)2, Ba(OH)2)
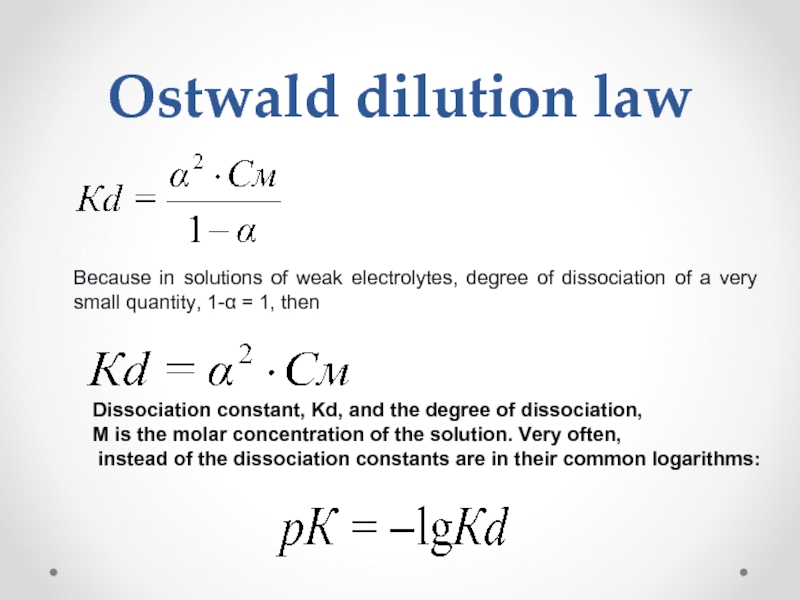
Слайд 33Ostwald dilution law
Because in solutions of weak electrolytes, degree of dissociation
Dissociation constant, Kd, and the degree of dissociation,
M is the molar concentration of the solution. Very often,
instead of the dissociation constants are in their common logarithms:
Слайд 34Acidity and basicity constants
The dissociation constants of acids and bases, respectively
Product constant acidity and basicity constants, with the acid conjugate base is the ion product of water:
Слайд 41Protolytic theory
Danish physicist and chemist Johannes Brønsted and the English chemist
Слайд 42
Base - a substance (particle) that can attach proton (i.e. base
Acid- a substance (particle) that can donate proton (i.e. acid – proton donor)
In the general form:
А-(acid); B-(base).
Such a system, consisting of acids and bases called protolytic conjugate pair of acid and base, offsetting or appropriate
Слайд 43Salt - the reaction product of acid and base
Example:
Conjugated acid
Conjugated base
By
Conjugated base
Conjugated acid
Слайд 44The homeostasis. The importancy of pH maintenance in human body
The human
Слайд 45The constancy of hydrogen ions concentration is one of important constant
1) Hydrogen ions have catalytic effect on many biochemical processes;
2)Enzymes and hormones exhibit biological activity only at a specific range of pH values;
3)Small changes of pH in blood and interstitial fluids affect the value of the osmotic pressure in this fluids.
Слайд 47The concept of buffer solutions
Buffer solutions are solutions that resist change
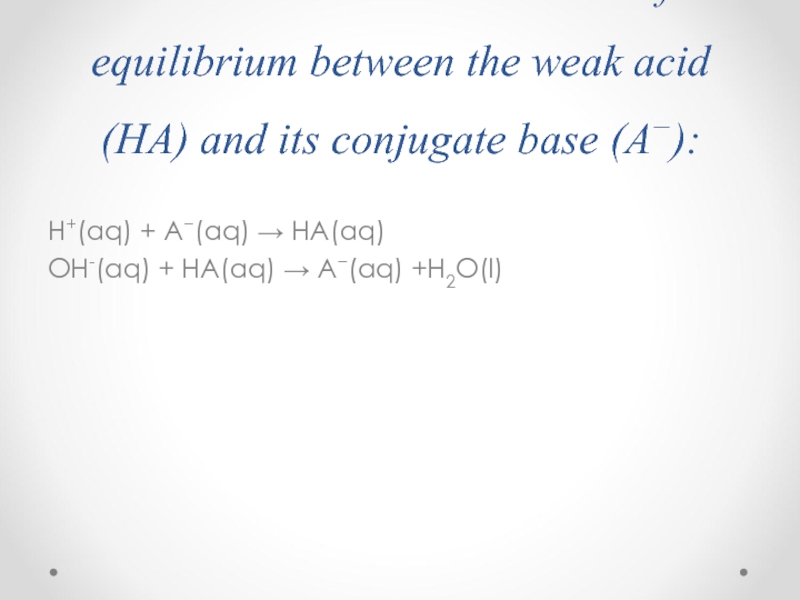
Слайд 49The resistive action is the result of the equilibrium between the
H+(aq) + A−(aq) → HA(aq)
OH-(aq) + HA(aq) → A−(aq) +H2O(l)
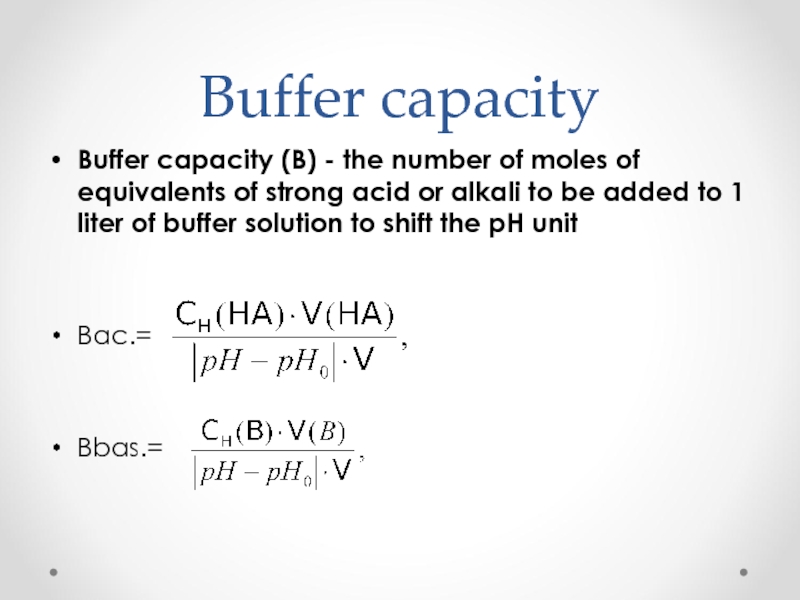
Слайд 51Buffer capacity
Buffer capacity (B) - the number of moles of equivalents
Вac.=
Вbas.=
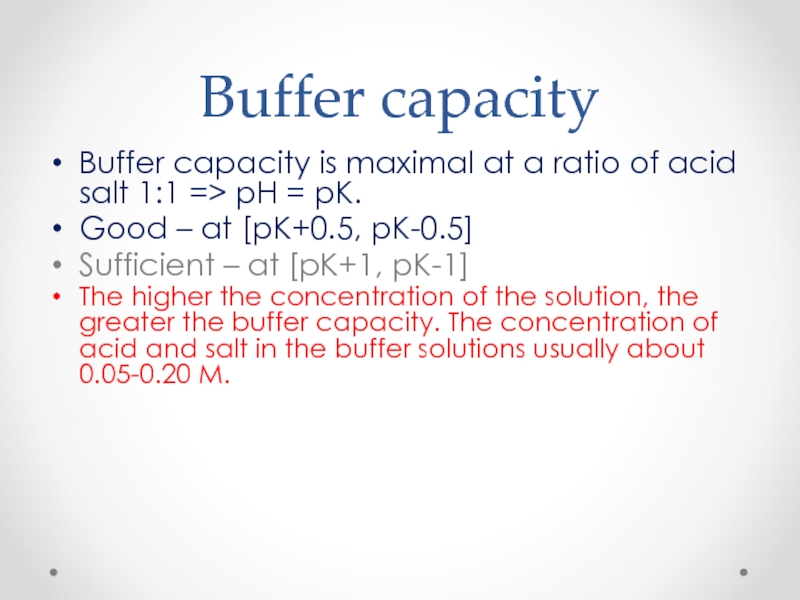
Слайд 52Buffer capacity
Buffer capacity is maximal at a ratio of acid salt
Good – at [pK+0.5, pK-0.5]
Sufficient – at [pK+1, pK-1]
The higher the concentration of the solution, the greater the buffer capacity. The concentration of acid and salt in the buffer solutions usually about 0.05-0.20 M.
Слайд 53
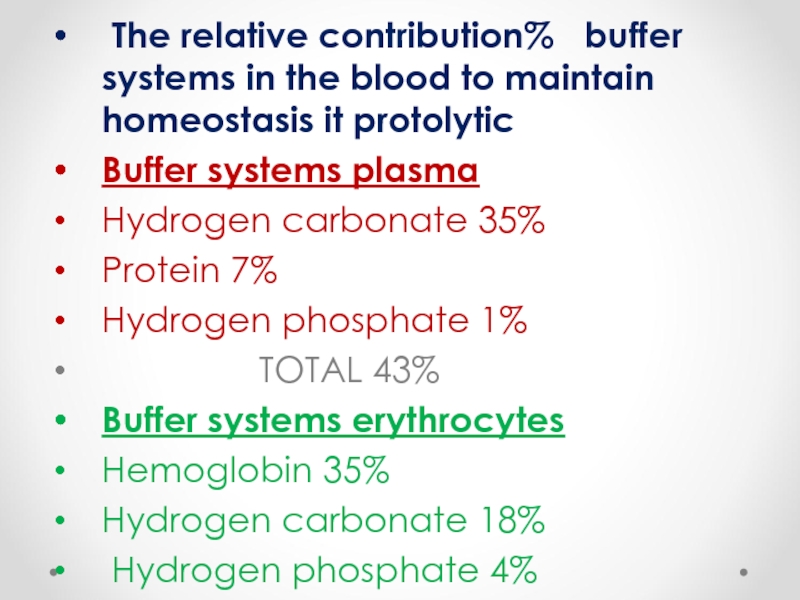
The relative contribution% buffer systems in the blood to
Buffer systems plasma
Hydrogen carbonate 35%
Protein 7%
Hydrogen phosphate 1%
TOTAL 43%
Buffer systems erythrocytes
Hemoglobin 35%
Hydrogen carbonate 18%
Hydrogen phosphate 4%
Слайд 58
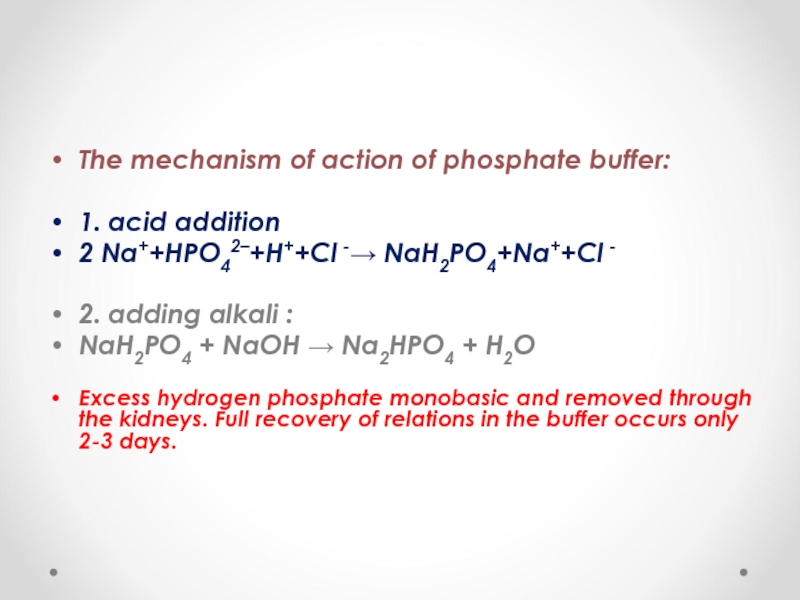
The mechanism of action of phosphate buffer:
1. acid addition
2 Na++HPO42–+H++Cl -→
2. adding alkali :
NaH2PO4 + NaOH → Na2HPO4 + H2O
Excess hydrogen phosphate monobasic and removed through the kidneys. Full recovery of relations in the buffer occurs only 2-3 days.
Слайд 60Protein buffer systems
The plasma proteins (albumins, globulins) are less important than
Слайд 66Hemoglobin buffer system
HHb + O2
Hemoglobin is a weaker acid (pKa HHb = 8.2) than oxyhemoglobin (pKa HHbO2 = 6.95). Therefore Hb- ions being anions of weaker acid are capable stronger to bind H+ ions than HbO2- ions.
Undissociated molecules HHbO2 lose O2 easier than the ions HbO2-
Слайд 67
a) the hemoglobin buffer system:
HHb
b) the buffer system formed by oxyhemoglobin:
HHbO2 H+ + HbO2-.
Слайд 76Literature
1. Medical Chemistry : textbook / V. A. Kalibabchuk [and al.] ;
2.http://www.chemeurope.com/en/encyclopedia/Buffer_solution.html
















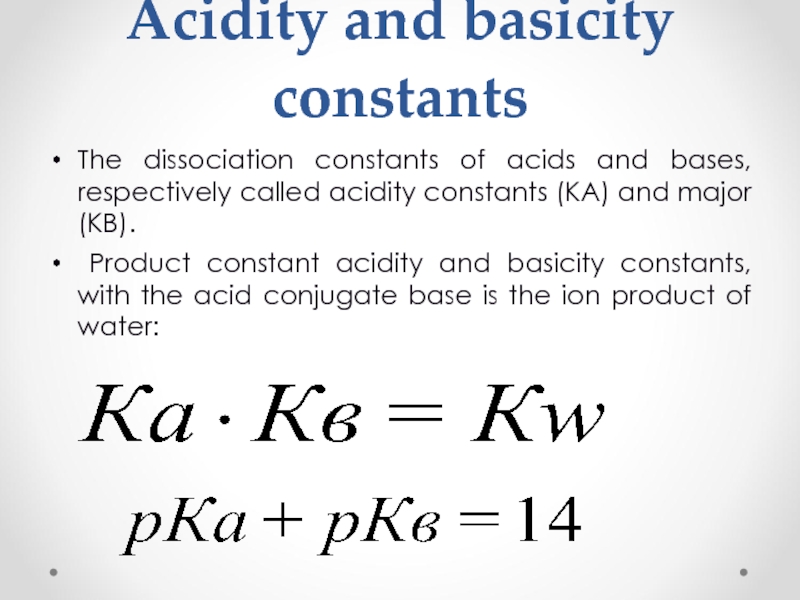
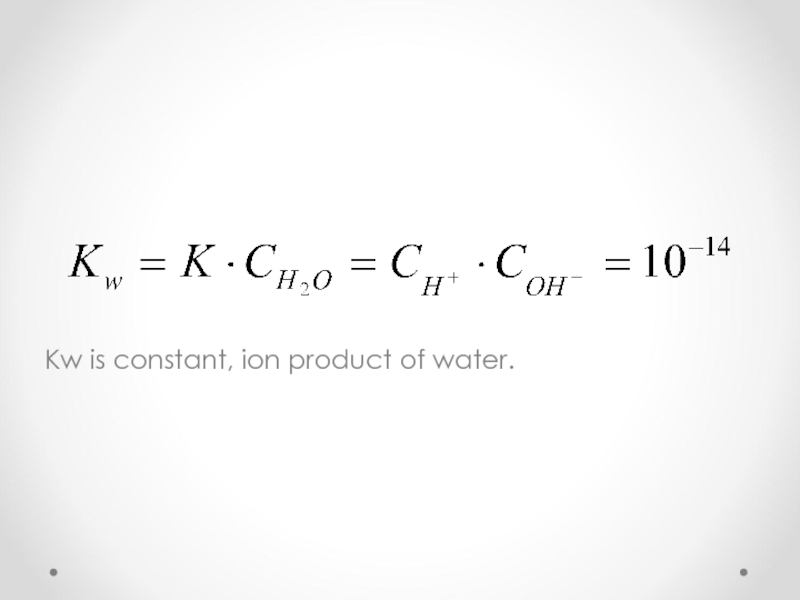
![Hydrogen ion exponentpH= -lg [H+]](/img/tmb/3/210152/c799d0193db64b750a8a47db4dae6148-800x.jpg)







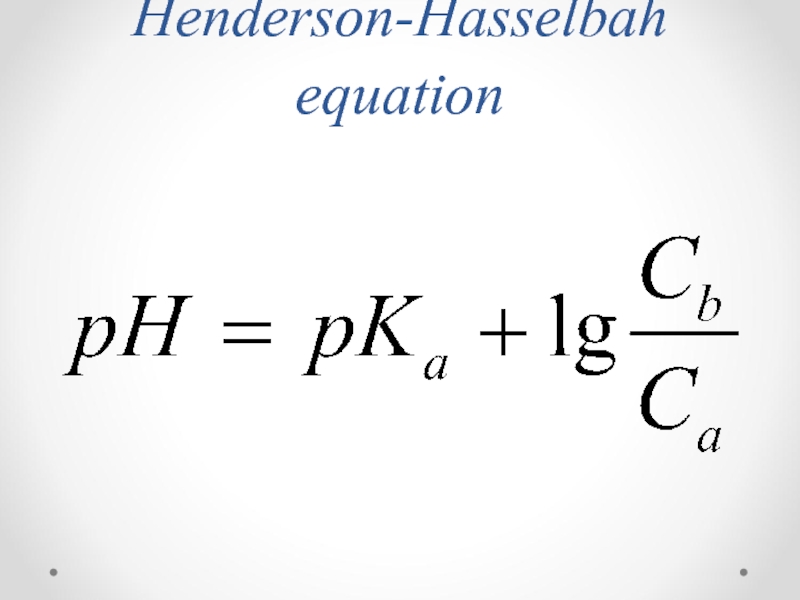











![Literature1. Medical Chemistry : textbook / V. A. Kalibabchuk [and al.] ; ed. by V. A.](/img/tmb/3/210152/1379e9ac7794809c421d5985d2014f8e-800x.jpg)





