- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Organic Compounds презентация
Содержание
- 2. Organic Compounds Containing Oxygen - III Session
- 3. Session Objectives Properties of phenols Reaction of
- 4. Acidity of phenol Phenol is more
- 5. Reactions of phenol Electrophilic aromatic substitution —OH
- 6. Chemical reaction of phenol Fries rearrangement Distillation with Zn dust :
- 7. Nitration With dilute HNO3, it gives
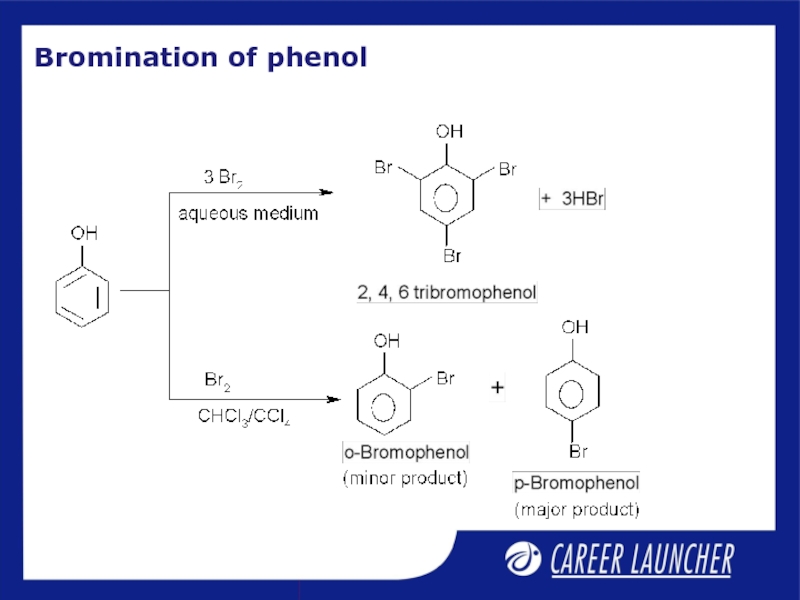
- 8. Bromination of phenol
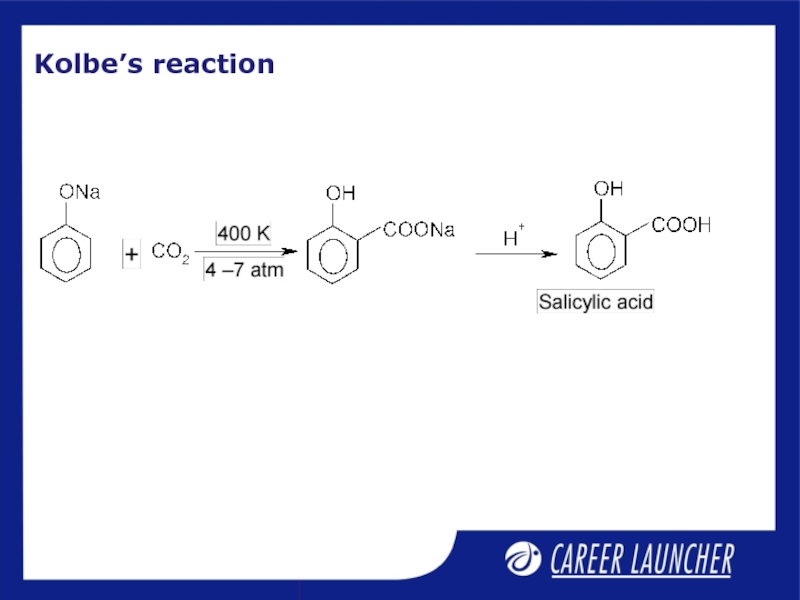
- 9. Kolbe’s reaction
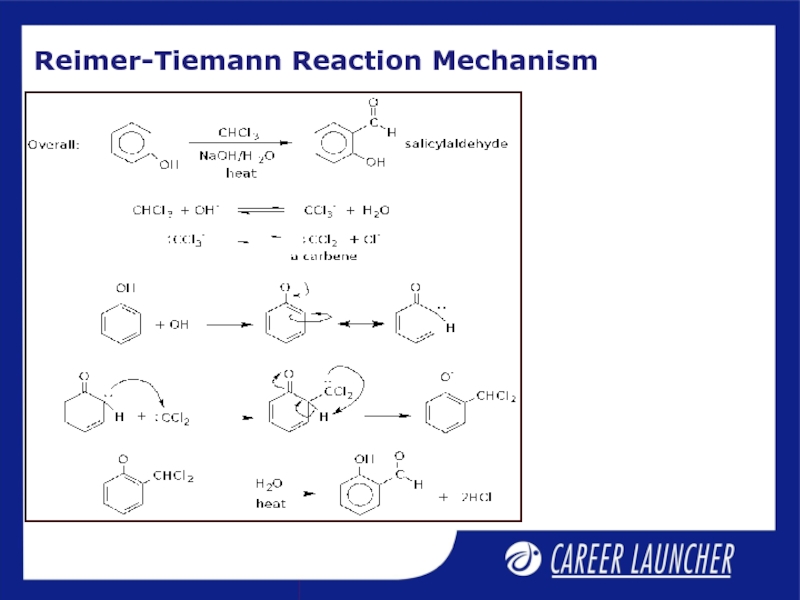
- 10. Reimer-Tiemann Reaction Mechanism
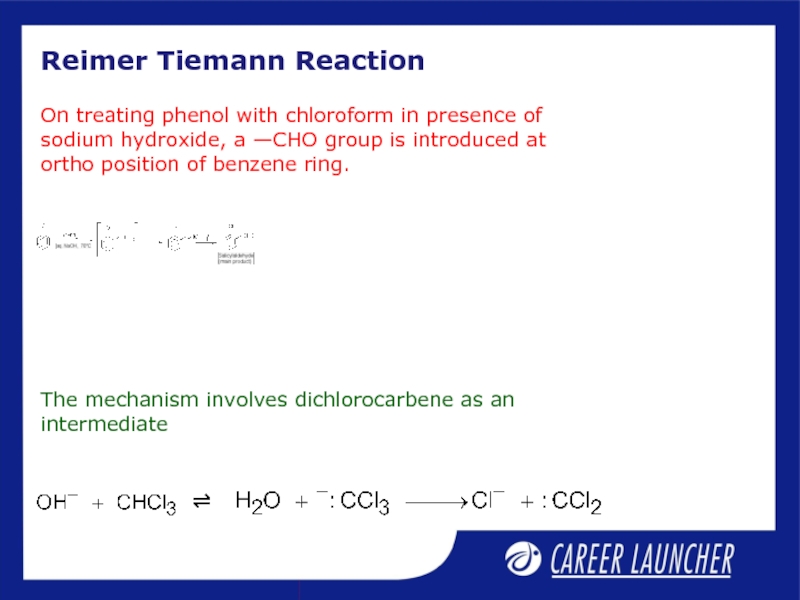
- 11. Reimer Tiemann Reaction The mechanism involves dichlorocarbene
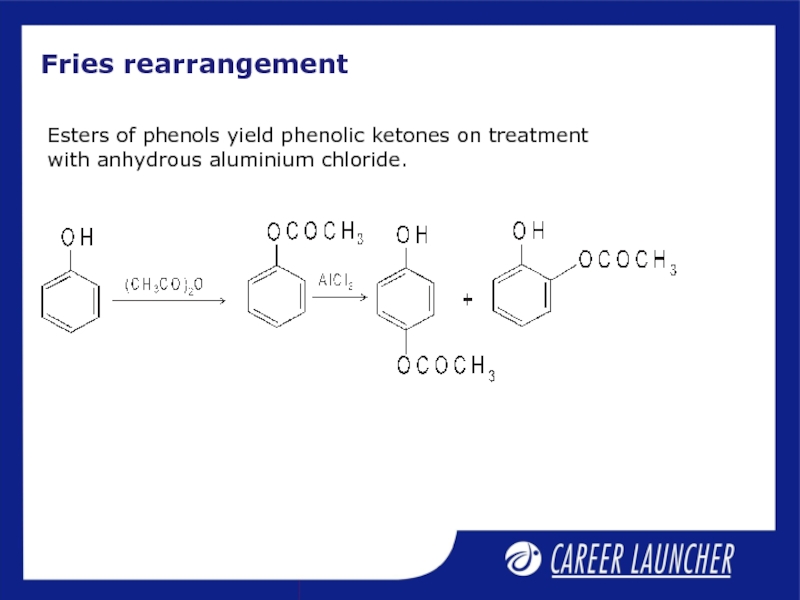
- 12. Fries rearrangement Esters of phenols yield phenolic ketones on treatment with anhydrous aluminium chloride.
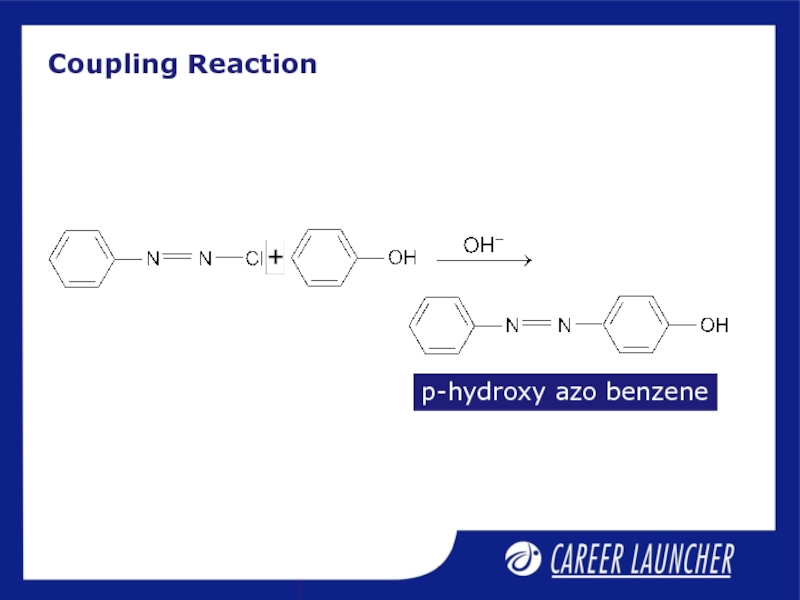
- 13. Coupling Reaction p-hydroxy azo benzene
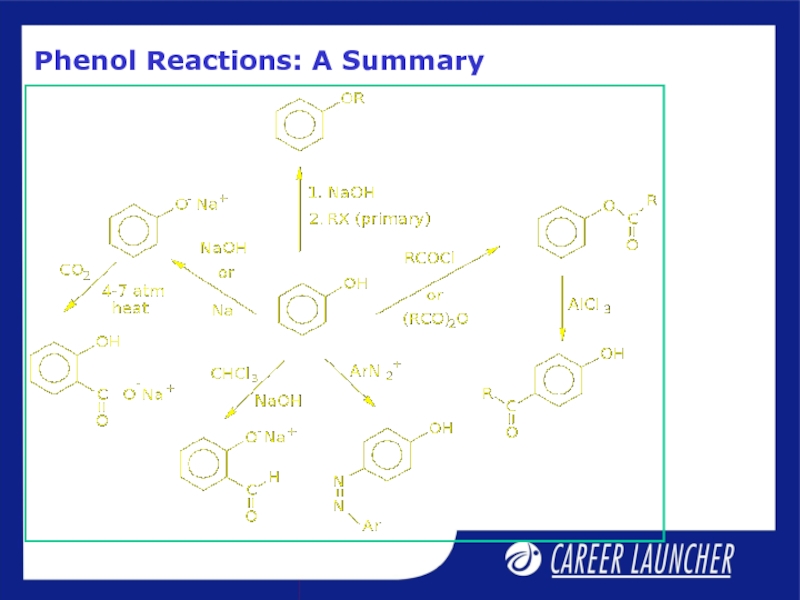
- 14. Phenol Reactions: A Summary
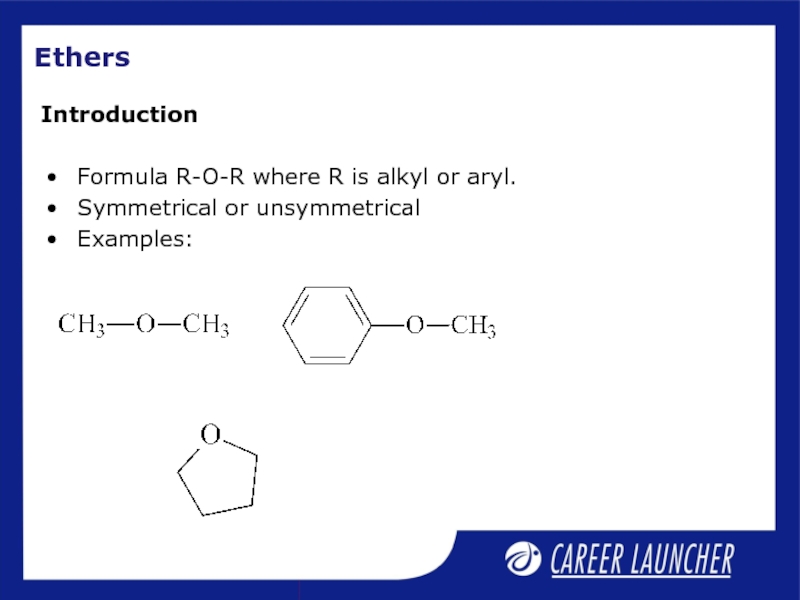
- 15. Ethers Formula R-O-R where R is alkyl or aryl. Symmetrical or unsymmetrical Examples: Introduction
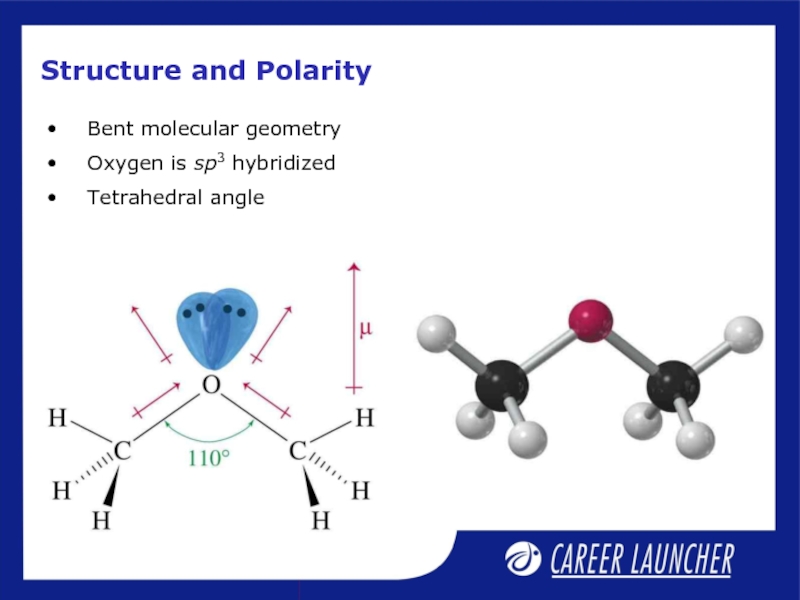
- 16. Structure and Polarity Bent molecular geometry Oxygen is sp3 hybridized Tetrahedral angle
- 17. Hydrogen Bond Acceptor Ethers cannot H-bond to
- 18. Solvent properties Nonpolar solutes dissolve better in
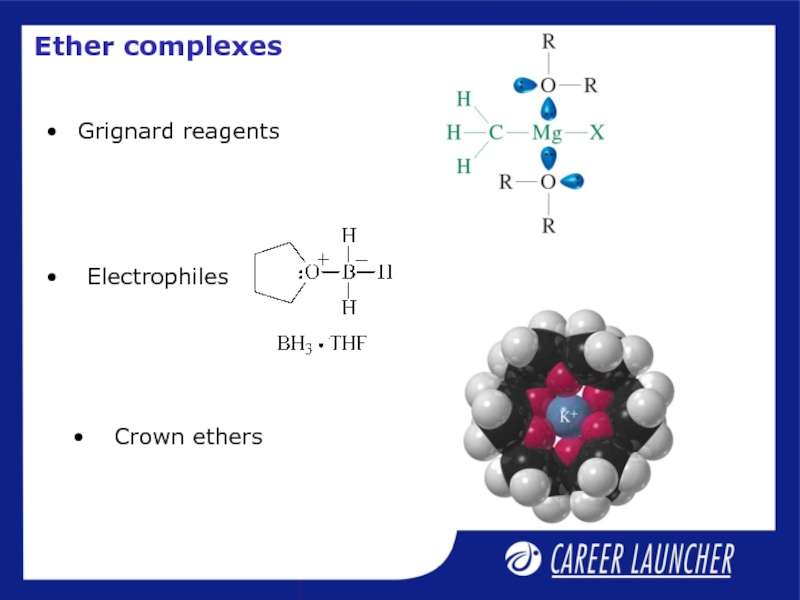
- 19. Grignard reagents Ether complexes Crown ethers Electrophiles
- 20. Nomenclature Alkyl alkyl ether Current rule:
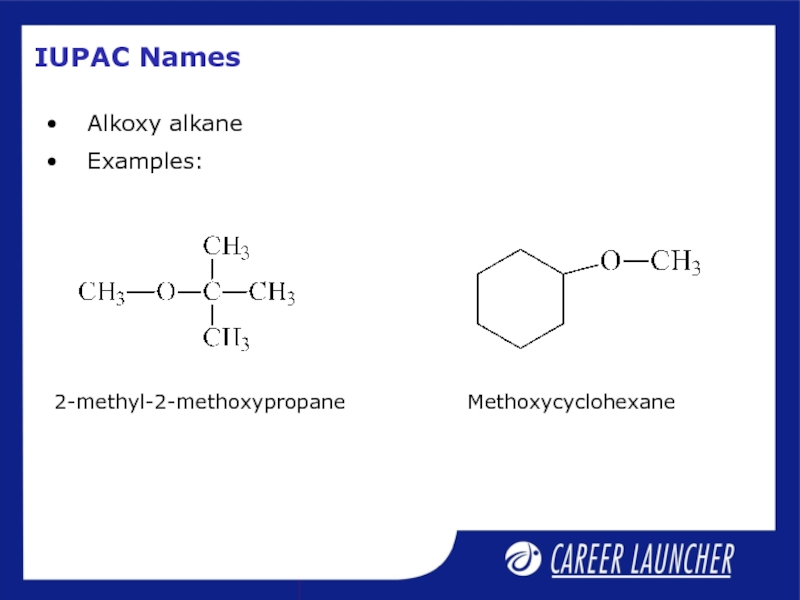
- 21. IUPAC Names Alkoxy alkane Examples:
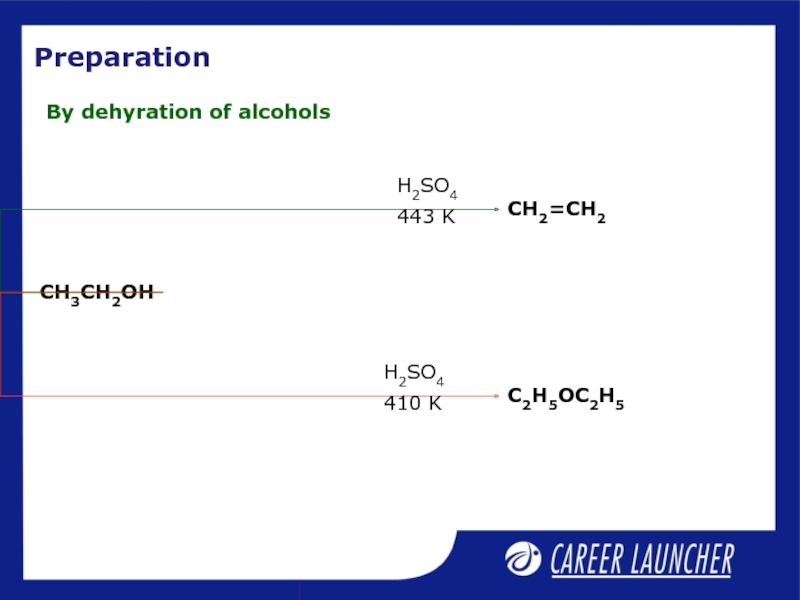
- 22. Preparation By dehyration of alcohols
- 23. Williamson’s Process Important laboratory method for the preparation of symmetrical and unsymmetrical ethers.
- 24. Williamsons Process Best results are obtained if
- 25. Cleavage of Ethers Ethers are unreactive toward
- 26. Phenyl Ether Cleavage Phenol cannot react further
- 27. Electrophilic substitution in alkyl aryl ethers The
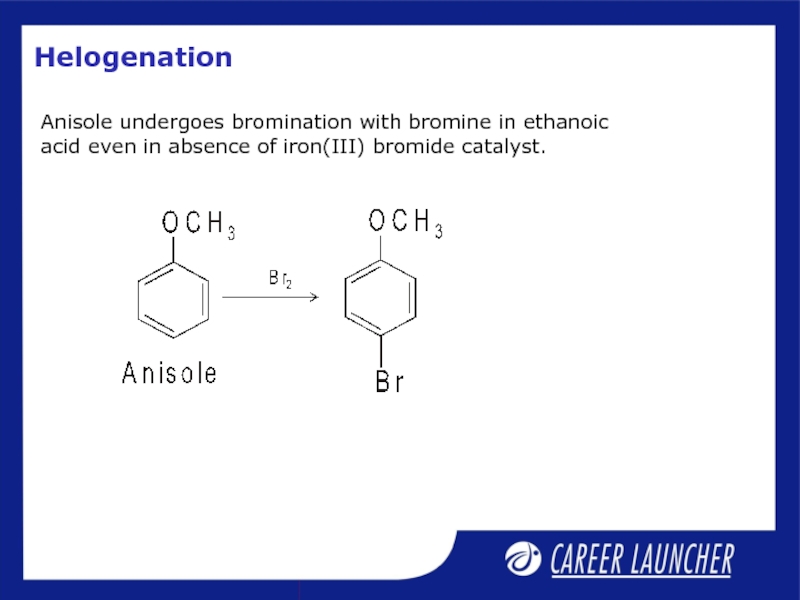
- 28. Helogenation Anisole undergoes bromination with bromine
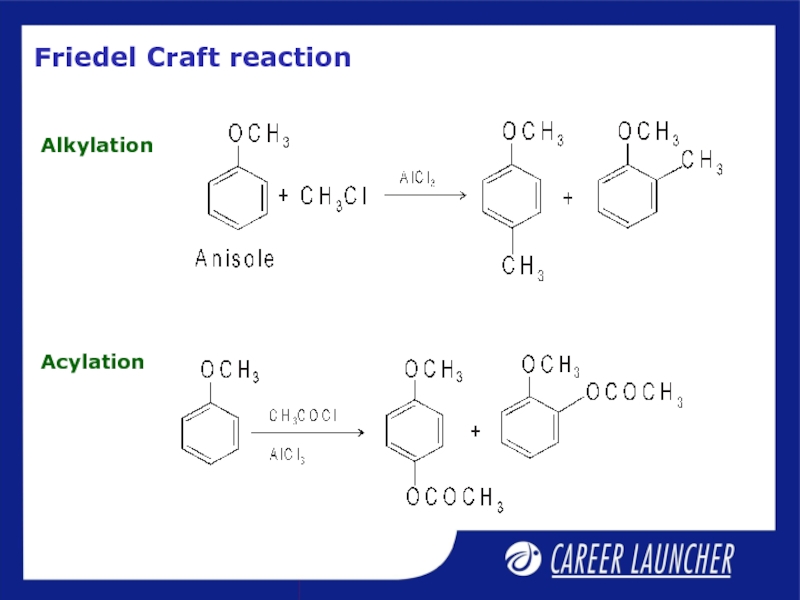
- 29. Friedel Craft reaction Alkylation Acylation
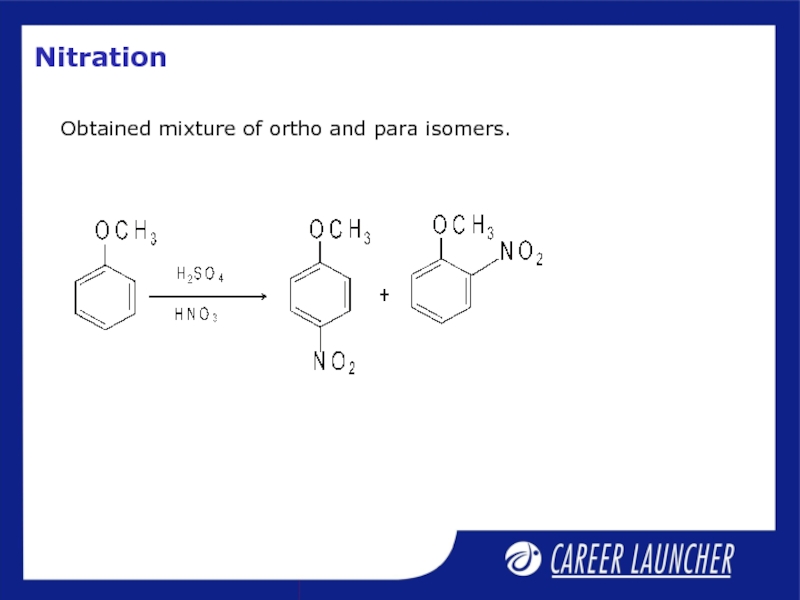
- 30. Nitration Obtained mixture of ortho and para isomers.
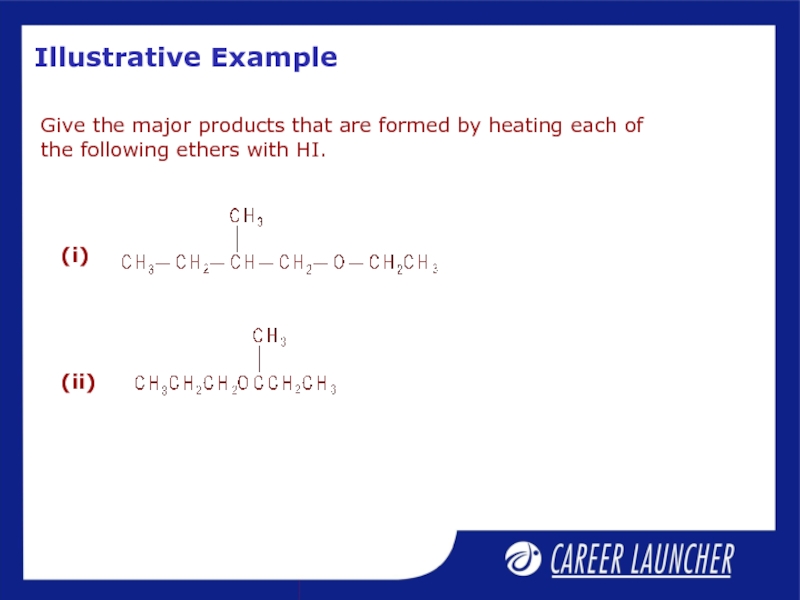
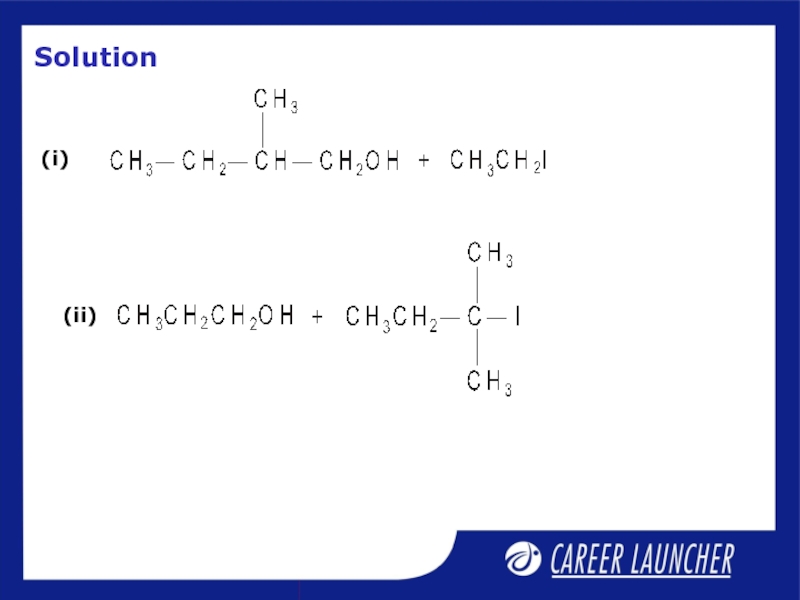
- 31. Illustrative Example
- 32. Solution
- 33. Crown ethers Cyclic polyethers containing four or
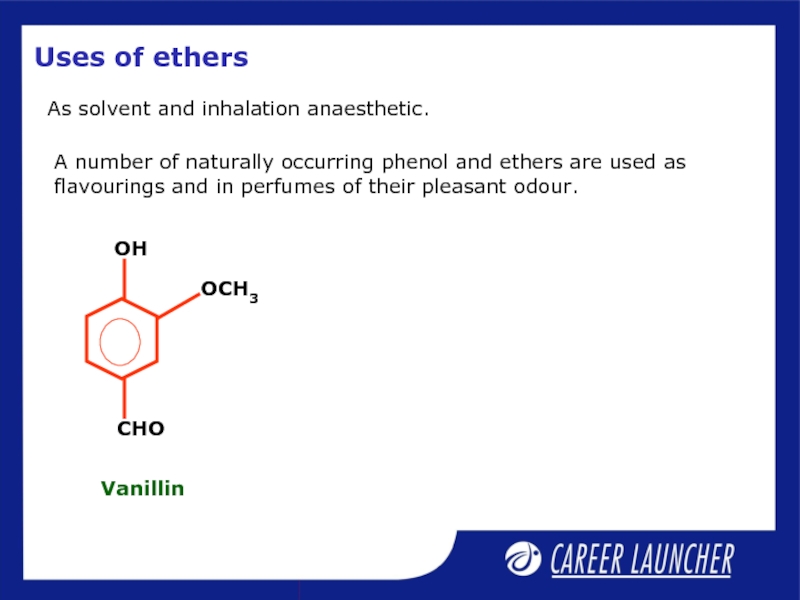
- 34. Uses of ethers As solvent and
Слайд 3Session Objectives
Properties of phenols
Reaction of phenols
Preparation of ethers
Properties and reactions of
Some useful ethers
Crown ethers
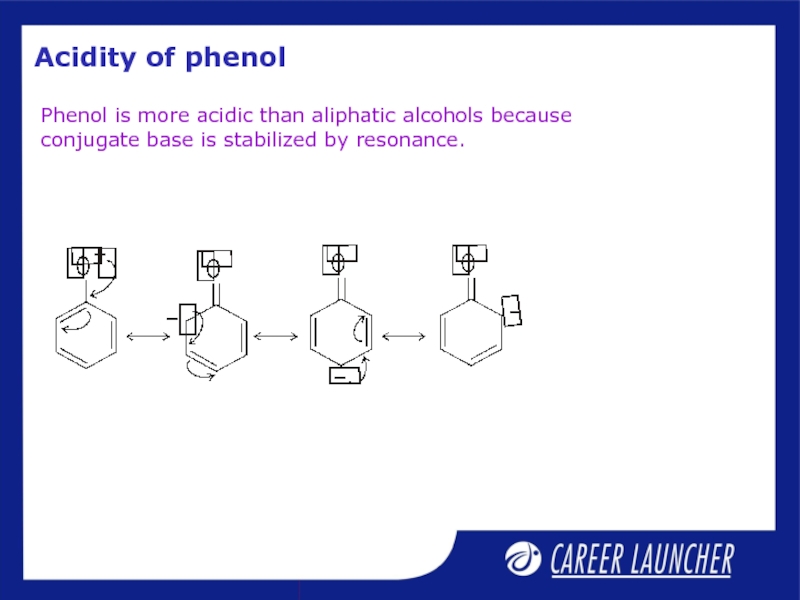
Слайд 4Acidity of phenol
Phenol is more acidic than aliphatic alcohols because conjugate
Слайд 5Reactions of phenol
Electrophilic aromatic substitution
—OH group is ortho, para- directing group
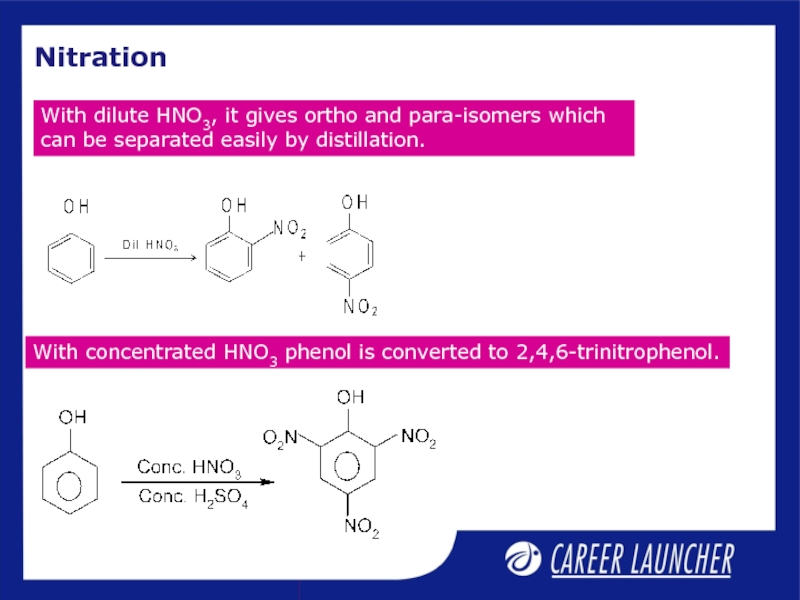
Слайд 7Nitration
With dilute HNO3, it gives ortho and para-isomers which can
With concentrated HNO3 phenol is converted to 2,4,6-trinitrophenol.
Слайд 11Reimer Tiemann Reaction
The mechanism involves dichlorocarbene as an
intermediate
On treating
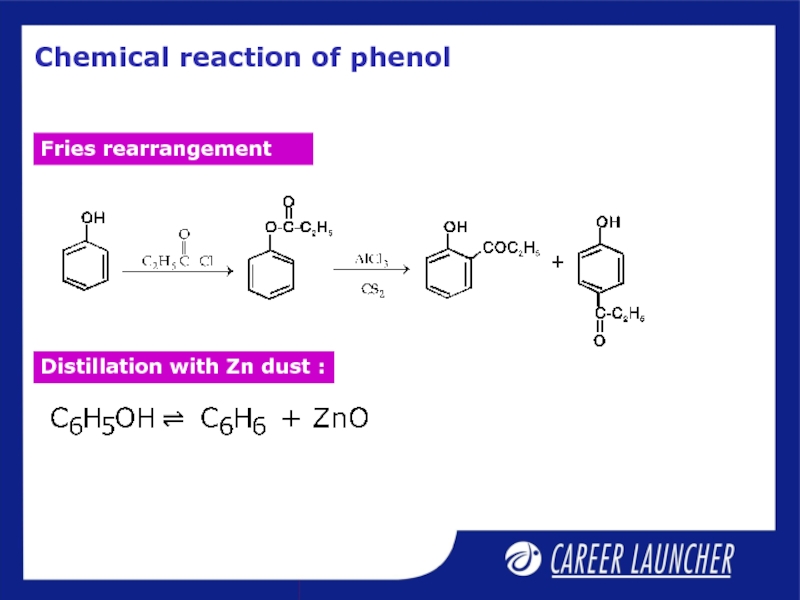
Слайд 12Fries rearrangement
Esters of phenols yield phenolic ketones on treatment with anhydrous
Слайд 15Ethers
Formula R-O-R where R is alkyl or aryl.
Symmetrical or unsymmetrical
Examples:
Introduction
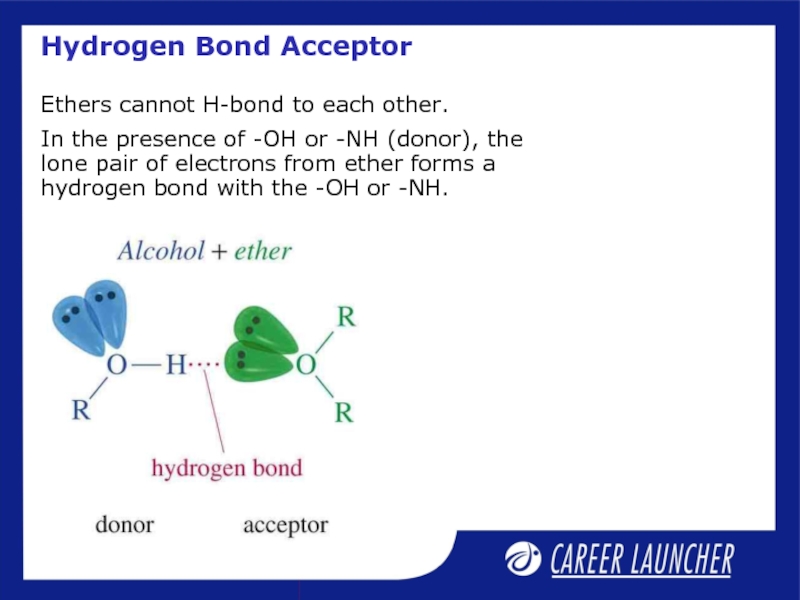
Слайд 17Hydrogen Bond Acceptor
Ethers cannot H-bond to each other.
In the presence of
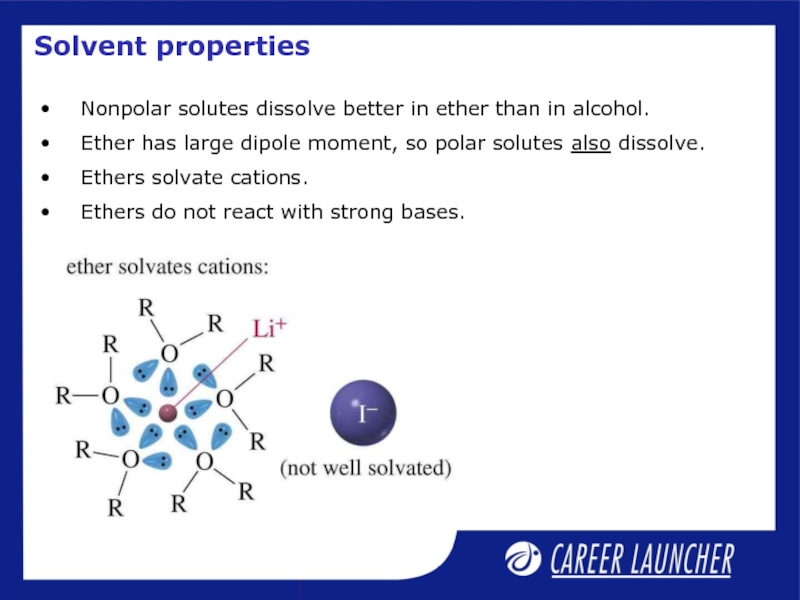
Слайд 18Solvent properties
Nonpolar solutes dissolve better in ether than in alcohol.
Ether has
Ethers solvate cations.
Ethers do not react with strong bases.
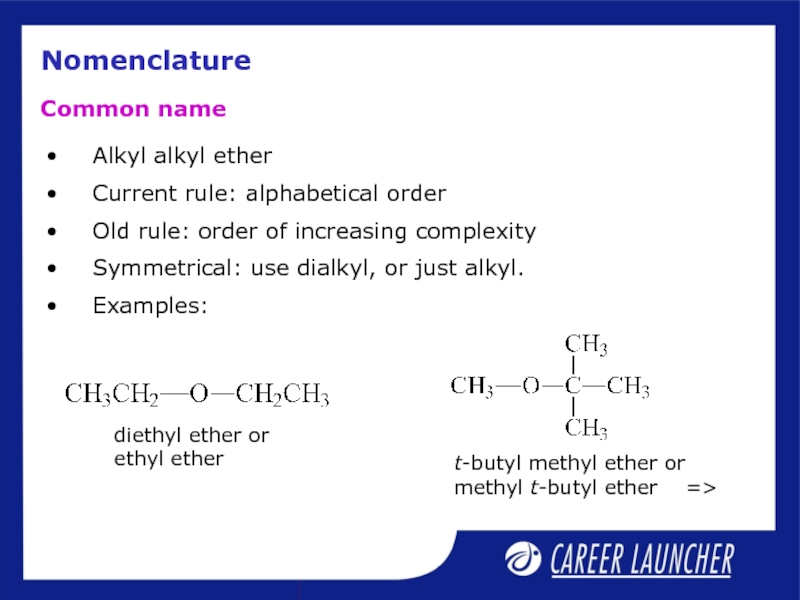
Слайд 20Nomenclature
Alkyl alkyl ether
Current rule: alphabetical order
Old rule: order of increasing
Symmetrical: use dialkyl, or just alkyl.
Examples:
Common name
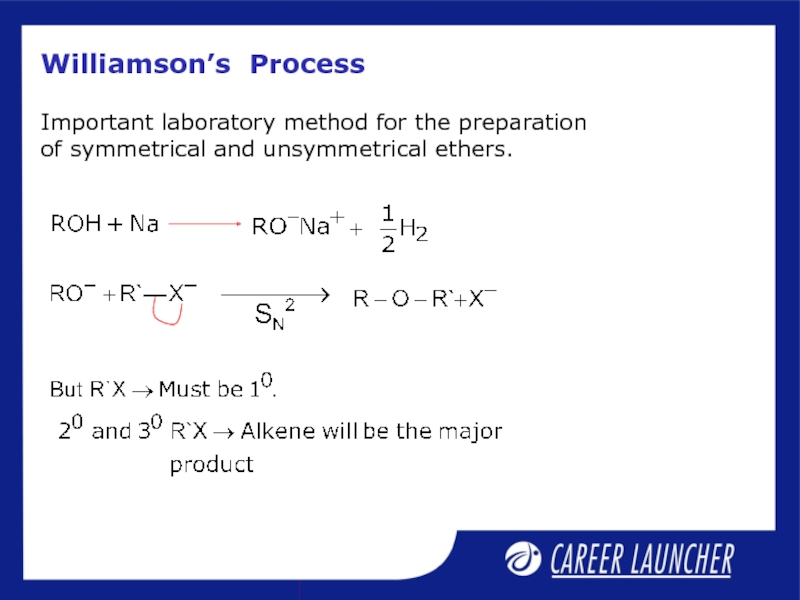
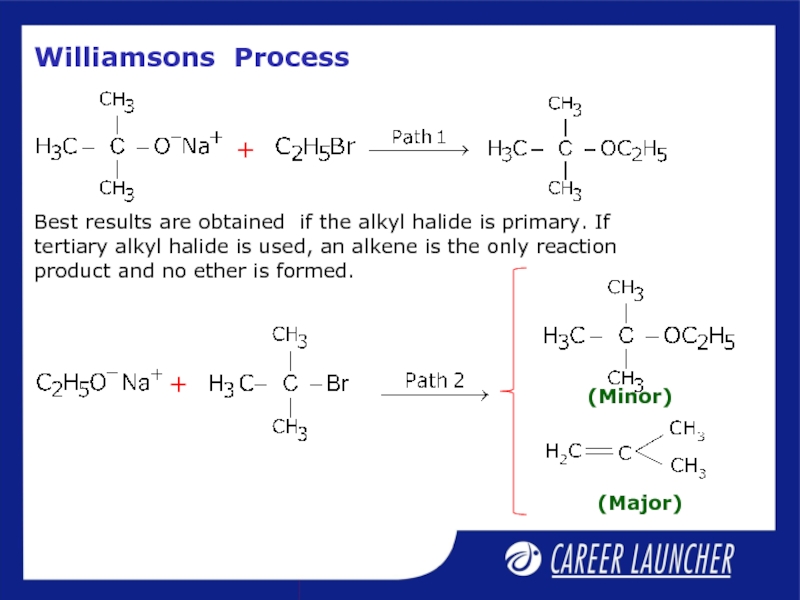
Слайд 23Williamson’s Process
Important laboratory method for the preparation of symmetrical and unsymmetrical
Слайд 24Williamsons Process
Best results are obtained if the alkyl halide is primary.
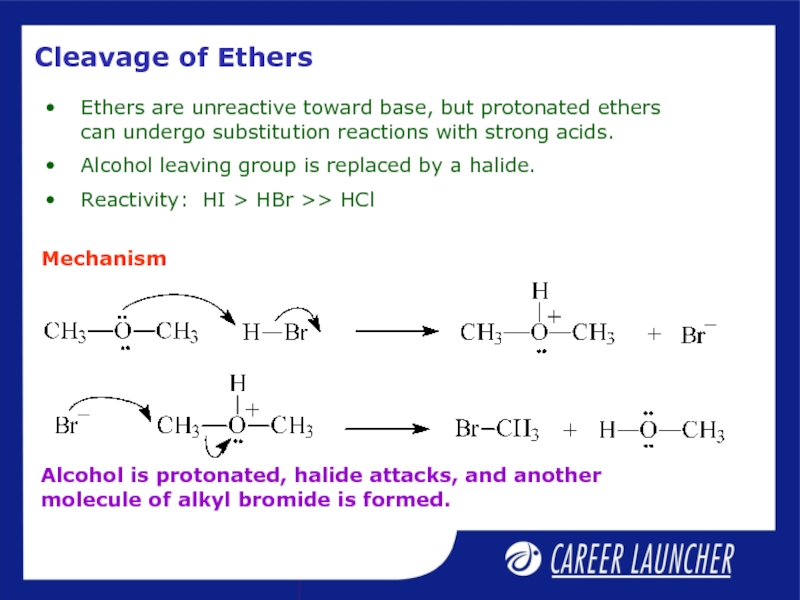
Слайд 25Cleavage of Ethers
Ethers are unreactive toward base, but protonated ethers can
Alcohol leaving group is replaced by a halide.
Reactivity: HI > HBr >> HCl
Mechanism
Alcohol is protonated, halide attacks, and another molecule of alkyl bromide is formed.
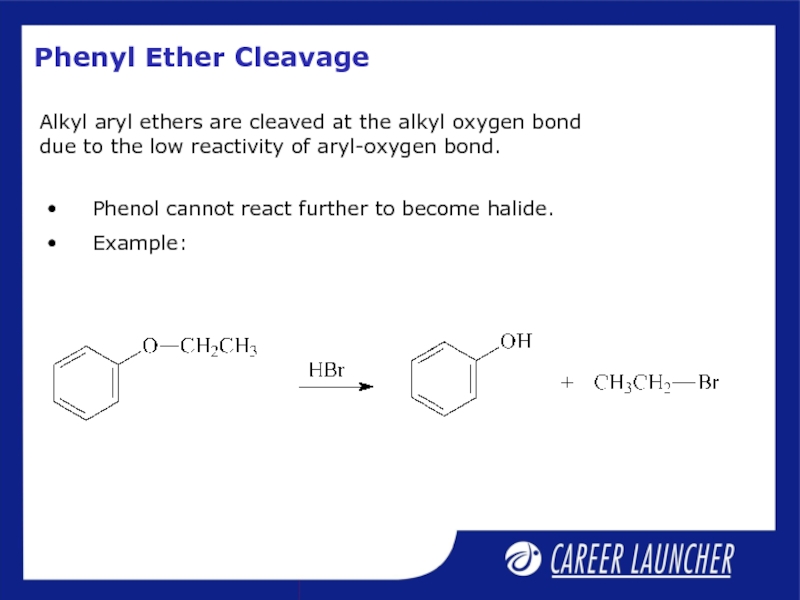
Слайд 26Phenyl Ether Cleavage
Phenol cannot react further to become halide.
Example:
Alkyl aryl ethers
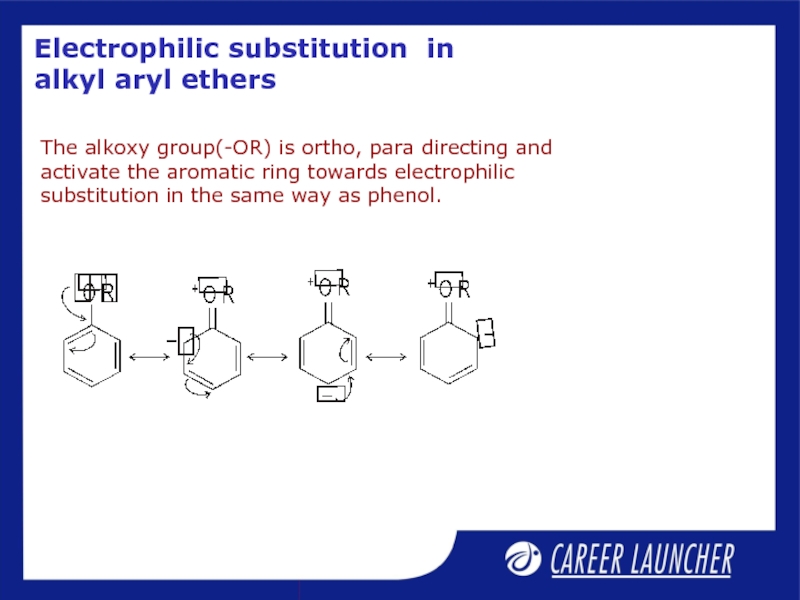
Слайд 27Electrophilic substitution in alkyl aryl ethers
The alkoxy group(-OR) is ortho, para
Слайд 28Helogenation
Anisole undergoes bromination with bromine in ethanoic acid even in
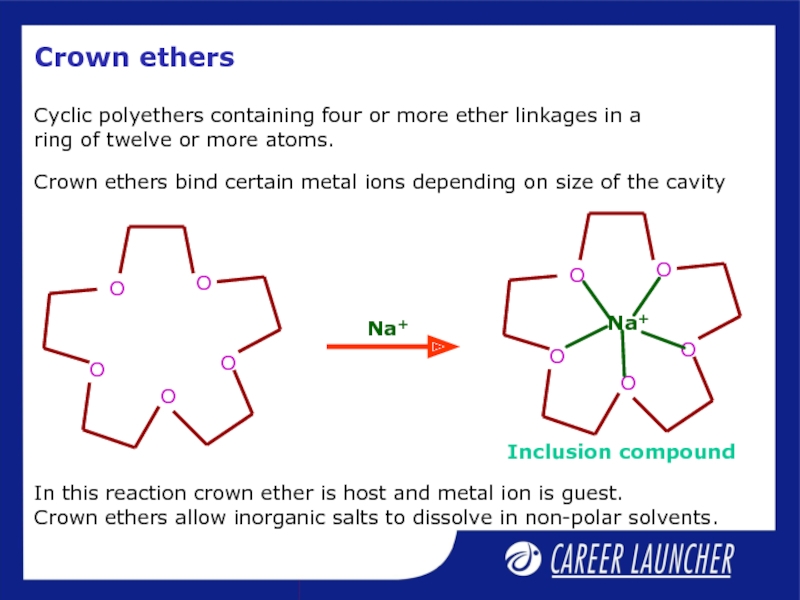
Слайд 33Crown ethers
Cyclic polyethers containing four or more ether linkages in a
Crown ethers bind certain metal ions depending on size of the cavity
In this reaction crown ether is host and metal ion is guest.
Crown ethers allow inorganic salts to dissolve in non-polar solvents.