Vitalievna
2016
- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Colligative properties of solutions презентация
Содержание
- 1. Colligative properties of solutions
- 2. Properties of solutions that depend on
- 3. Raoult’s Law: Fractional lowering of the
- 4. The freezing point of a nonvolatile substance
- 5. Spontaneous process of solute concentration leveling in
- 6. One-side diffusion of solvent molecules through a
- 7. Van't Hoff ‘s Law (1887): Osmotic pressure
- 8. Turgor is a state
- 10. С1
Слайд 1 Zaporizhzhya State Medical University Analytical Chemistry Department COLLIGATIVE PROPERTIES OF SOLUTIONS Lecturer: Monaykina Yulia
Слайд 2
Properties of solutions that depend on the number of molecules present
and not on the kind of molecules are called colligative properties.
These properties include
vapor pressure depression,
boiling point elevation,
freezing point depression,
diffusion and osmotic pressure.
These properties include
vapor pressure depression,
boiling point elevation,
freezing point depression,
diffusion and osmotic pressure.
Слайд 3Raoult’s Law:
Fractional lowering of the saturated vapor pressure of a
solvent above a solution is equal to the mole fraction of the dissolved substance:
Слайд 4The freezing point of a nonvolatile substance solution is always lower
than the freezing point of a solvent.
And the boiling point of a nonvolatile substance solution is always higher than the freezing point of a solvent.
And the boiling point of a nonvolatile substance solution is always higher than the freezing point of a solvent.
Слайд 5Spontaneous process of solute concentration leveling in the whole volume of
the solution, due to the thermal motion of the solute and solvent is called diffusion.
Diffusion can also occur if a semipermeable membrane that could allow only molecules of the solvent is a boundary between solution and pure solvent (or two solutions of different concentrations).
Many natural films (the intestinal wall, protoplasm, etc.) have properties of semipermeable membranes.
Слайд 6One-side diffusion of solvent molecules through a semipermeable membrane to a
more concentrated solution is called osmosis.
Osmotic pressure is the external pressure on a solution, at which osmotic equilibrium (through a semipermeable membrane) between the solution and a pure solvent is established.
Osmotic pressure is the external pressure on a solution, at which osmotic equilibrium (through a semipermeable membrane) between the solution and a pure solvent is established.
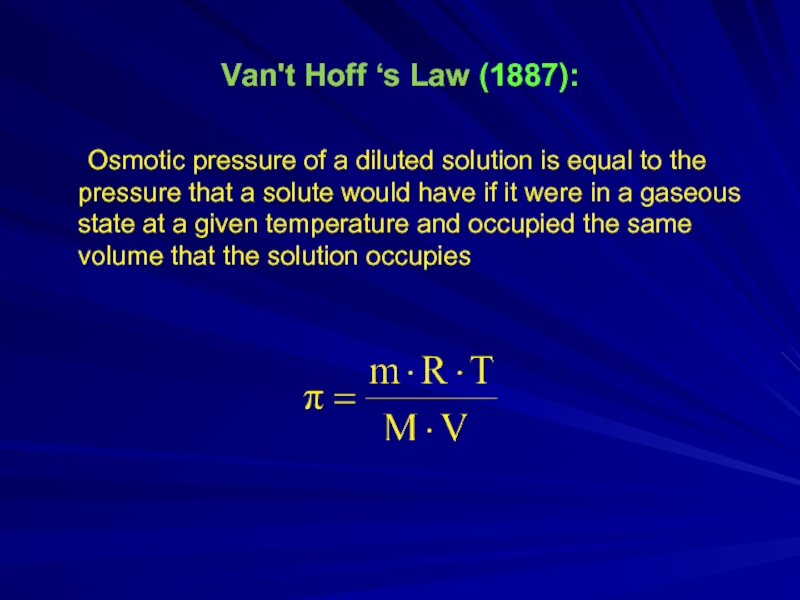
Слайд 7Van't Hoff ‘s Law (1887):
Osmotic pressure of a diluted solution is
equal to the pressure that a solute would have if it were in a gaseous state at a given temperature and occupied the same volume that the solution occupies
Слайд 8
Turgor is a state of tension of the cellular
cover caused by osmotic pressure of the cell contents.
Turgor supports tissue elasticity and resiliency, promotes certain form of organs.
Turgor supports tissue elasticity and resiliency, promotes certain form of organs.
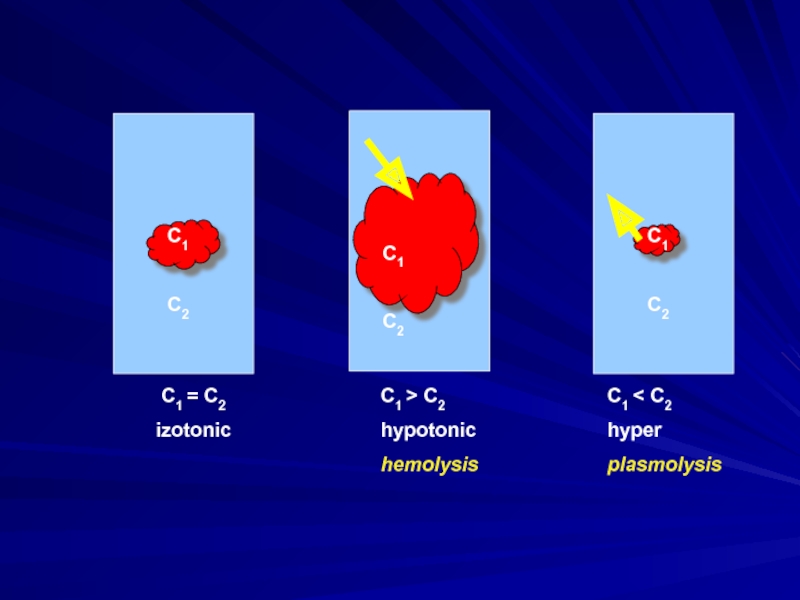
Слайд 9 Solutions with an identical osmotic pressure are called isotonic. Solutions with
a higher osmotic pressure than that of a solution of comparison are called hypertonic.
Solutions with a lower osmotic pressure are hypotonic.