- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
An Introduction to Metabolism презентация
Содержание
- 1. An Introduction to Metabolism
- 2. Overview: The Energy of Life The living
- 3. Fig. 8-1
- 4. Concept 8.1: An organism’s metabolism transforms matter
- 5. Organization of the Chemistry of Life into
- 6. Fig. 8-UN1 Enzyme 1 Enzyme 2 Enzyme
- 7. Catabolic pathways release energy by breaking down
- 8. Anabolic pathways consume energy to build complex
- 9. Forms of Energy Energy is the capacity
- 10. Kinetic energy is energy associated with motion
- 11. Fig. 8-2 Climbing up converts the kinetic
- 12. The Laws of Energy Transformation Thermodynamics is
- 13. The First Law of Thermodynamics According to
- 14. The Second Law of Thermodynamics During every
- 15. Fig. 8-3 (a) First law of thermodynamics
- 16. Living cells unavoidably convert organized forms of
- 17. Biological Order and Disorder Cells create ordered
- 18. Fig. 8-4 50 µm
- 19. The evolution of more complex organisms does
- 20. Concept 8.2: The free-energy change of a
- 21. Free-Energy Change, ΔG A living system’s free
- 22. The change in free energy (∆G) during
- 23. Free Energy, Stability, and Equilibrium Free energy
- 24. Fig. 8-5 (a) Gravitational motion (b) Diffusion
- 25. Fig. 8-5a Less free energy (lower
- 26. Fig. 8-5b Spontaneous change Spontaneous change Spontaneous
- 27. Free Energy and Metabolism The concept of
- 28. Exergonic and Endergonic Reactions in Metabolism An
- 29. Fig. 8-6 Reactants Energy Free energy Products
- 30. Fig. 8-6a Energy (a) Exergonic reaction: energy
- 31. Fig. 8-6b Energy (b) Endergonic reaction: energy
- 32. Equilibrium and Metabolism Reactions in a closed
- 33. Fig. 8-7 (a) An isolated hydroelectric system
- 34. Fig. 8-7a (a) An isolated hydroelectric system ∆G < 0 ∆G = 0
- 35. Fig. 8-7b (b) An open hydroelectric system ∆G < 0
- 36. Fig. 8-7c (c) A multistep open hydroelectric
- 37. Concept 8.3: ATP powers cellular work by
- 38. The Structure and Hydrolysis of ATP ATP
- 39. Fig. 8-8 Phosphate groups Ribose Adenine
- 40. The bonds between the phosphate groups of
- 41. Fig. 8-9 Inorganic phosphate Energy Adenosine triphosphate
- 42. How ATP Performs Work The three types
- 43. Fig. 8-10 (b) Coupled with ATP hydrolysis,
- 44. ATP drives endergonic reactions by phosphorylation, transferring
- 45. Fig. 8-11 (b) Mechanical work: ATP binds
- 46. The Regeneration of ATP ATP is a
- 47. Fig. 8-12 P i ADP + Energy
- 48. Concept 8.4: Enzymes speed up metabolic reactions
- 49. Fig. 8-13 Sucrose (C12H22O11) Glucose (C6H12O6) Fructose (C6H12O6) Sucrase
- 50. The Activation Energy Barrier Every chemical reaction
- 51. Fig. 8-14 Progress of the reaction Products
- 52. How Enzymes Lower the EA Barrier Enzymes
- 53. Fig. 8-15 Progress of the reaction Products
- 54. Substrate Specificity of Enzymes The reactant that
- 55. Fig. 8-16 Substrate Active site Enzyme Enzyme-substrate complex (b) (a)
- 56. Catalysis in the Enzyme’s Active Site In
- 57. Fig. 8-17 Substrates Enzyme Products are released.
- 58. Effects of Local Conditions on Enzyme Activity
- 59. Effects of Temperature and pH Each enzyme
- 60. Fig. 8-18 Rate of reaction Optimal temperature
- 61. Cofactors Cofactors are nonprotein enzyme helpers Cofactors
- 62. Enzyme Inhibitors Competitive inhibitors bind to the
- 63. Fig. 8-19 (a) Normal binding (c) Noncompetitive
- 64. Concept 8.5: Regulation of enzyme activity helps
- 65. Allosteric Regulation of Enzymes Allosteric regulation may
- 66. Allosteric Activation and Inhibition Most allosterically regulated
- 67. Fig. 8-20 Allosteric enyzme with four subunits
- 68. Fig. 8-20a (a) Allosteric activators and inhibitors
- 69. Cooperativity is a form of allosteric regulation
- 70. Fig. 8-20b (b) Cooperativity: another type of
- 71. Identification of Allosteric Regulators Allosteric regulators are
- 72. Fig. 8-21 RESULTS EXPERIMENT Caspase
- 73. Fig. 8-21a SH Substrate Hypothesis: allosteric
- 74. Fig. 8-21b Caspase 1 RESULTS Active form Inhibitor Allosterically inhibited form Inactive form
- 75. Feedback Inhibition In feedback inhibition, the end
- 76. Fig. 8-22 Intermediate C Feedback inhibition Isoleucine
- 77. Specific Localization of Enzymes Within the Cell
- 78. Fig. 8-23 1 µm Mitochondria
- 79. Fig. 8-UN2 Progress of the reaction Products
- 80. Fig. 8-UN3
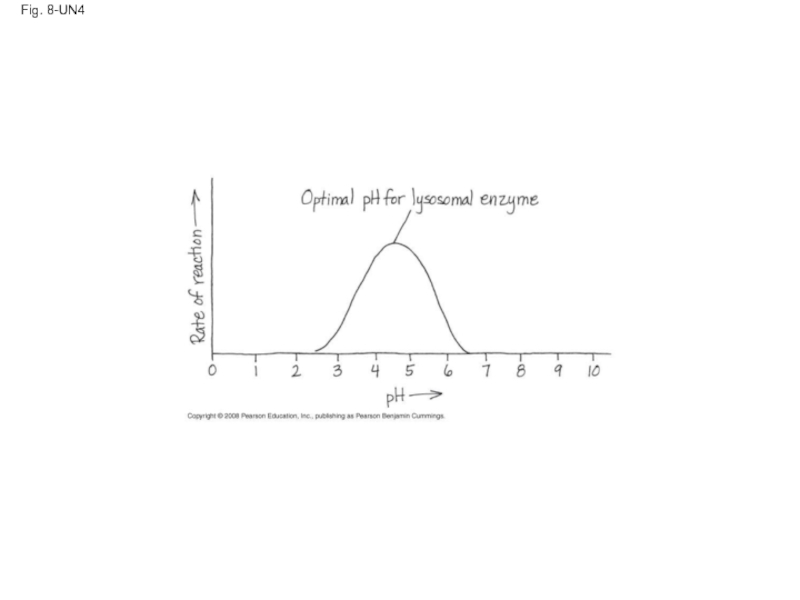
- 81. Fig. 8-UN4
- 82. Fig. 8-UN5
- 83. You should now be able to: Distinguish
- 84. Explain how ATP performs cellular work
Слайд 2Overview: The Energy of Life
The living cell is a miniature chemical
The cell extracts energy and applies energy to perform work
Some organisms even convert energy to light, as in bioluminescence
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 4Concept 8.1: An organism’s metabolism transforms matter and energy, subject to
Metabolism is the totality of an organism’s chemical reactions
Metabolism is an emergent property of life that arises from interactions between molecules within the cell
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 5Organization of the Chemistry of Life into Metabolic Pathways
A metabolic pathway
Each step is catalyzed by a specific enzyme
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 7Catabolic pathways release energy by breaking down complex molecules into simpler
Cellular respiration, the breakdown of glucose in the presence of oxygen, is an example of a pathway of catabolism
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
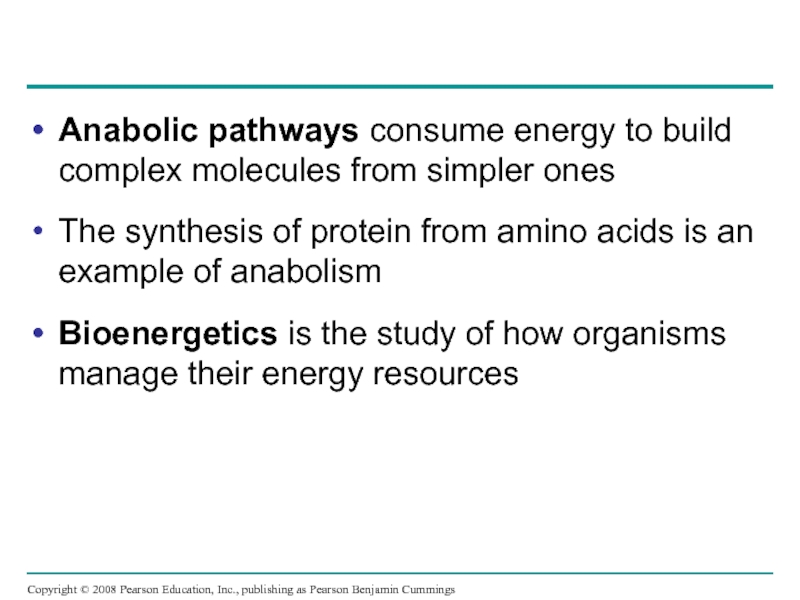
Слайд 8Anabolic pathways consume energy to build complex molecules from simpler ones
The
Bioenergetics is the study of how organisms manage their energy resources
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
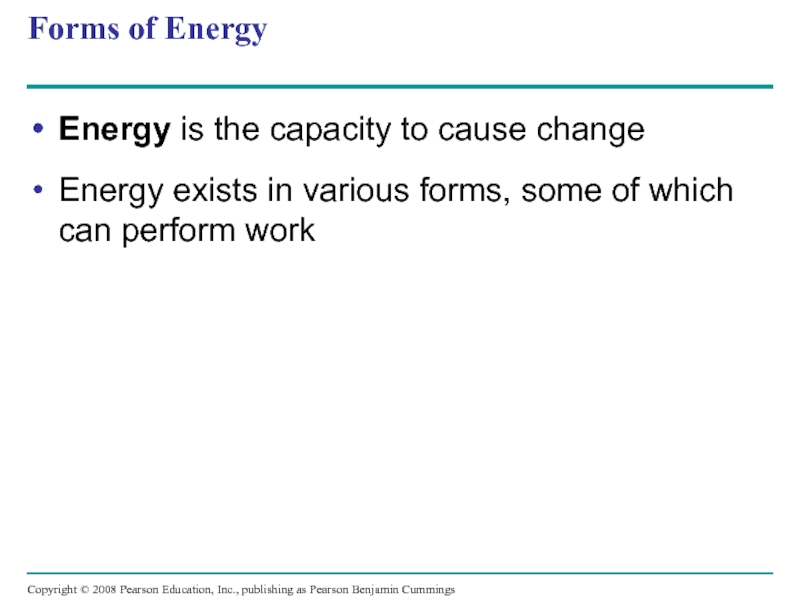
Слайд 9Forms of Energy
Energy is the capacity to cause change
Energy exists in
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
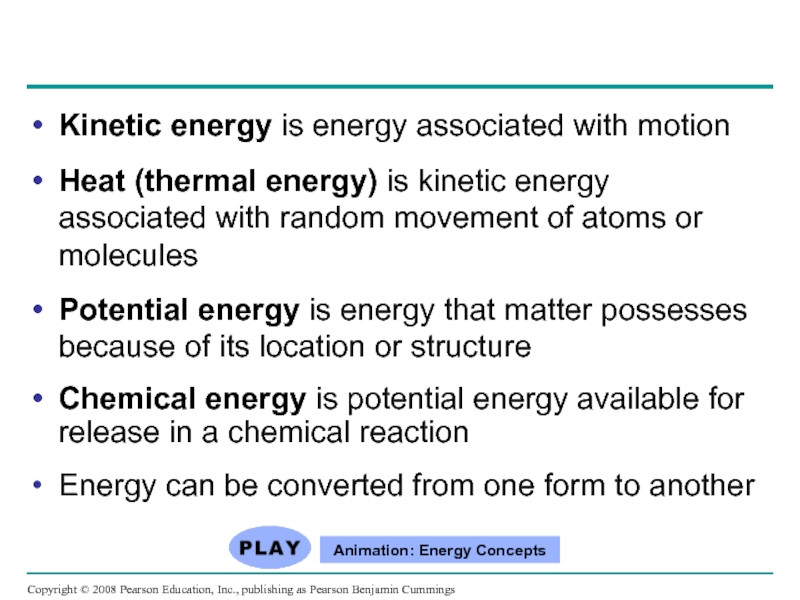
Слайд 10Kinetic energy is energy associated with motion
Heat (thermal energy) is kinetic
Potential energy is energy that matter possesses because of its location or structure
Chemical energy is potential energy available for release in a chemical reaction
Energy can be converted from one form to another
Animation: Energy Concepts
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 11Fig. 8-2
Climbing up converts the kinetic
energy of muscle movement
to potential energy.
A
energy in the water
than on the platform.
Diving converts
potential energy to
kinetic energy.
A diver has more potential
energy on the platform
than in the water.
Слайд 12The Laws of Energy Transformation
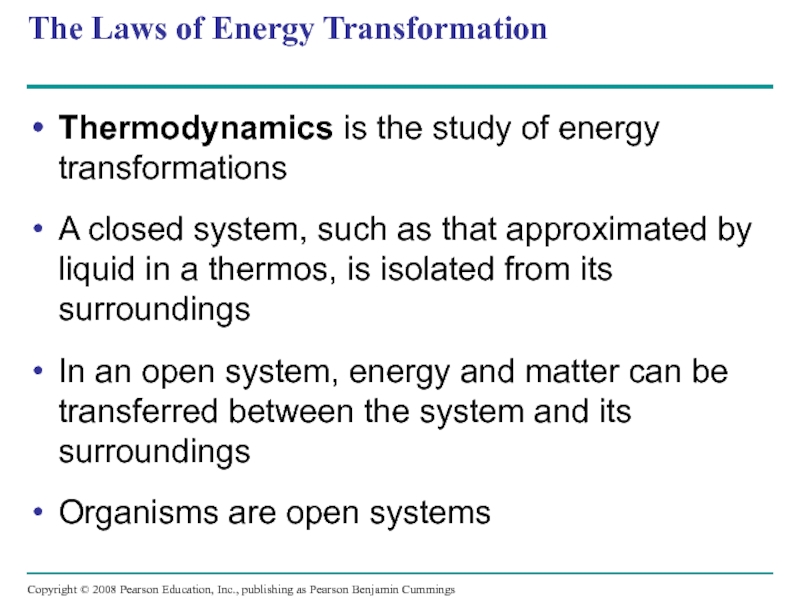
Thermodynamics is the study of energy transformations
A
In an open system, energy and matter can be transferred between the system and its surroundings
Organisms are open systems
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
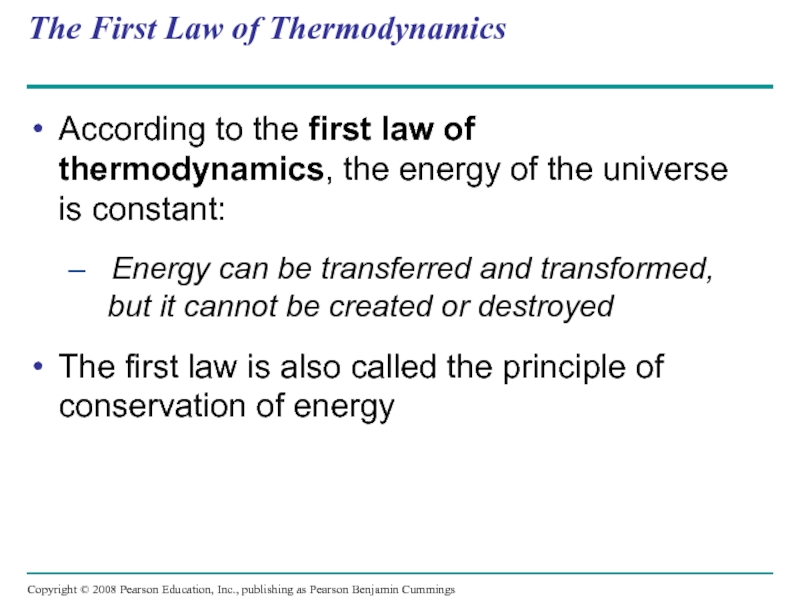
Слайд 13The First Law of Thermodynamics
According to the first law of thermodynamics,
– Energy can be transferred and transformed, but it cannot be created or destroyed
The first law is also called the principle of conservation of energy
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 14The Second Law of Thermodynamics
During every energy transfer or transformation, some
According to the second law of thermodynamics:
– Every energy transfer or transformation increases the entropy (disorder) of the universe
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
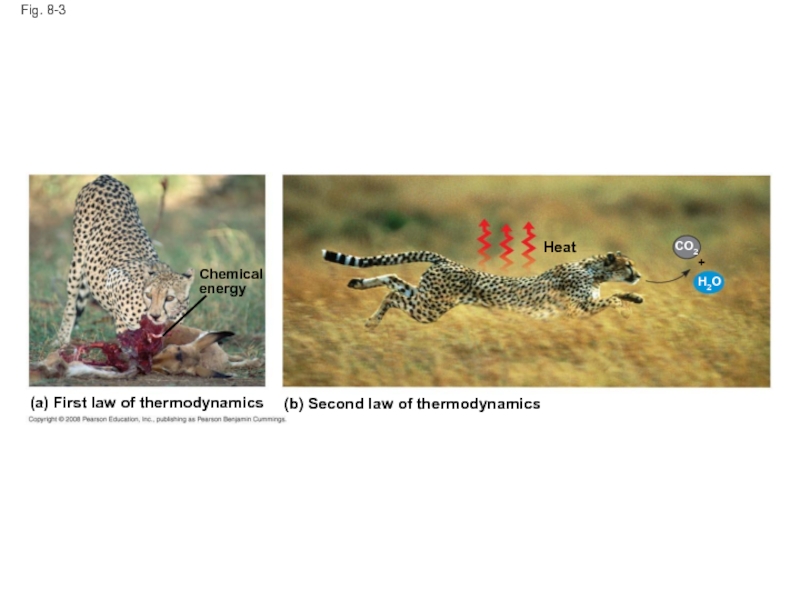
Слайд 15Fig. 8-3
(a) First law of thermodynamics
(b) Second law of thermodynamics
Chemical
energy
Heat
CO2
H2O
+
Слайд 16Living cells unavoidably convert organized forms of energy to heat
Spontaneous processes
For a process to occur without energy input, it must increase the entropy of the universe
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
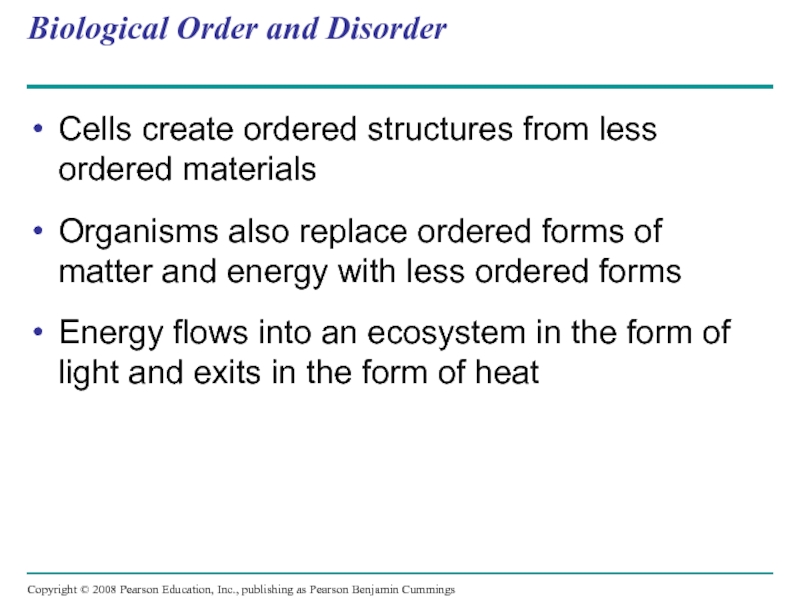
Слайд 17Biological Order and Disorder
Cells create ordered structures from less ordered materials
Organisms
Energy flows into an ecosystem in the form of light and exits in the form of heat
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
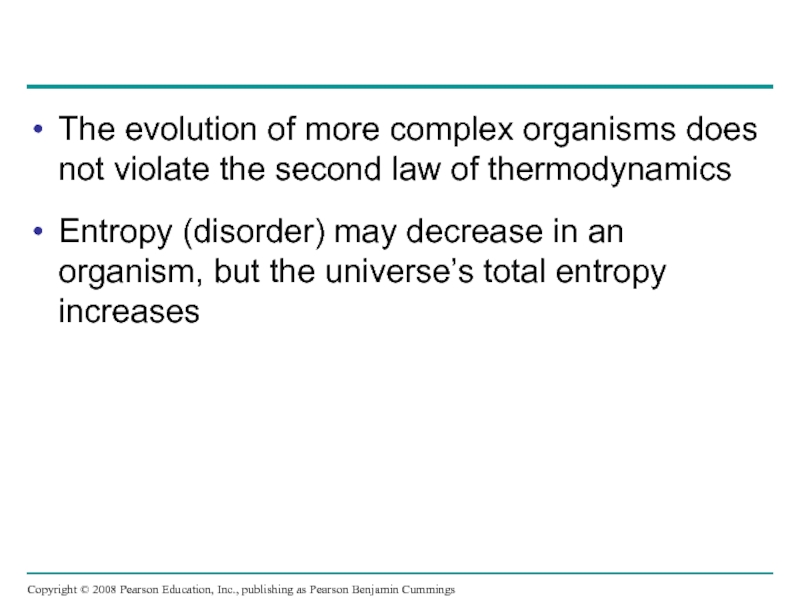
Слайд 19The evolution of more complex organisms does not violate the second
Entropy (disorder) may decrease in an organism, but the universe’s total entropy increases
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 20Concept 8.2: The free-energy change of a reaction tells us whether
Biologists want to know which reactions occur spontaneously and which require input of energy
To do so, they need to determine energy changes that occur in chemical reactions
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 21Free-Energy Change, ΔG
A living system’s free energy is energy that can
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
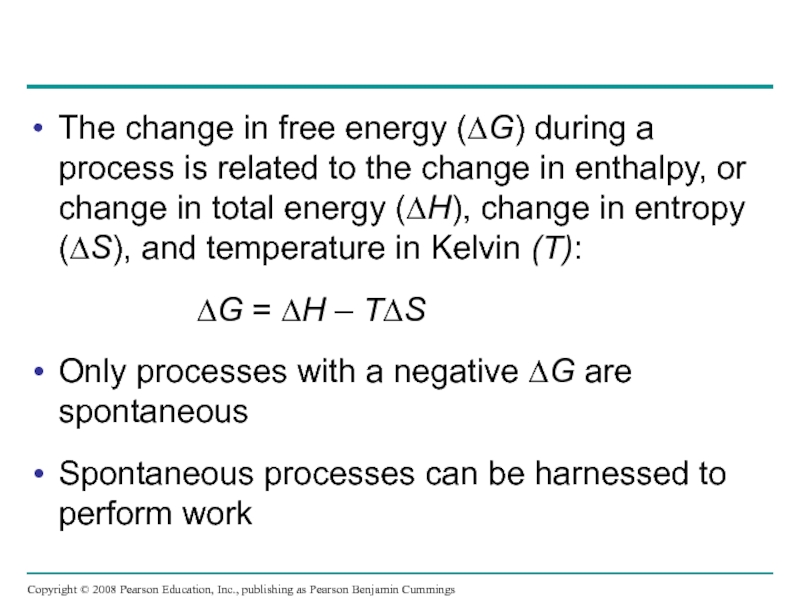
Слайд 22The change in free energy (∆G) during a process is related
∆G = ∆H – T∆S
Only processes with a negative ∆G are spontaneous
Spontaneous processes can be harnessed to perform work
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
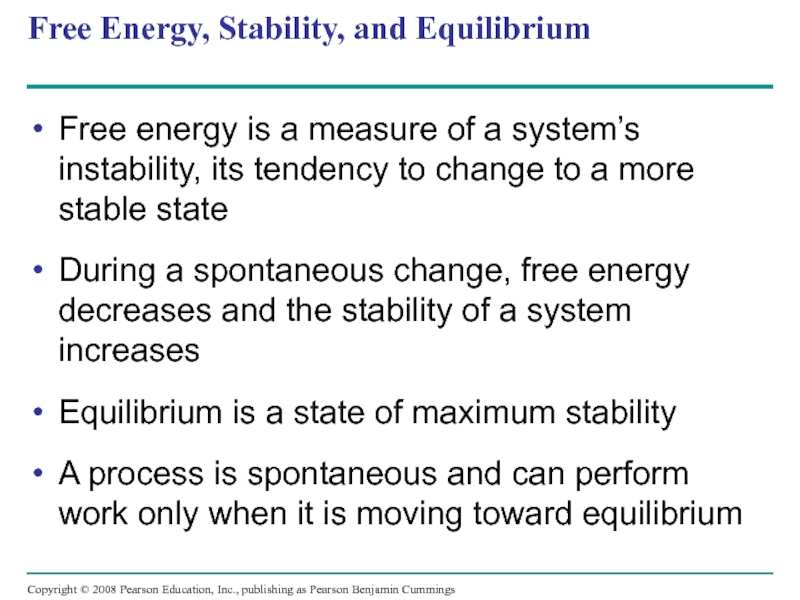
Слайд 23Free Energy, Stability, and Equilibrium
Free energy is a measure of a
During a spontaneous change, free energy decreases and the stability of a system increases
Equilibrium is a state of maximum stability
A process is spontaneous and can perform work only when it is moving toward equilibrium
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
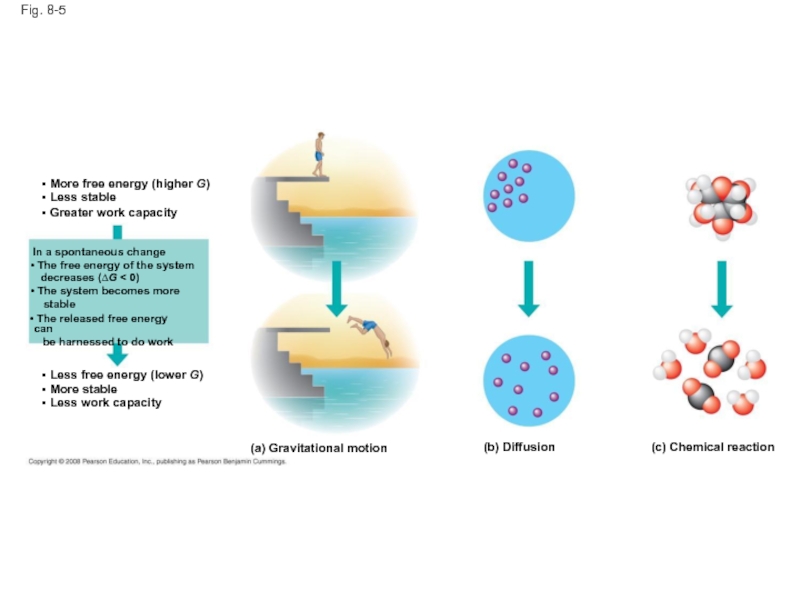
Слайд 24Fig. 8-5
(a) Gravitational motion
(b) Diffusion
(c) Chemical reaction
More free energy (higher
Less stable
Greater work capacity
In a spontaneous change
The free energy of the system
decreases (∆G < 0)
The system becomes more
stable
The released free energy can
be harnessed to do work
Less free energy (lower G)
More stable
Less work capacity
Слайд 25Fig. 8-5a
Less free energy (lower G)
More stable
Less work
More free energy (higher G)
Less stable
Greater work capacity
In a spontaneous change
The free energy of the system
decreases (∆G < 0)
The system becomes more
stable
The released free energy can
be harnessed to do work
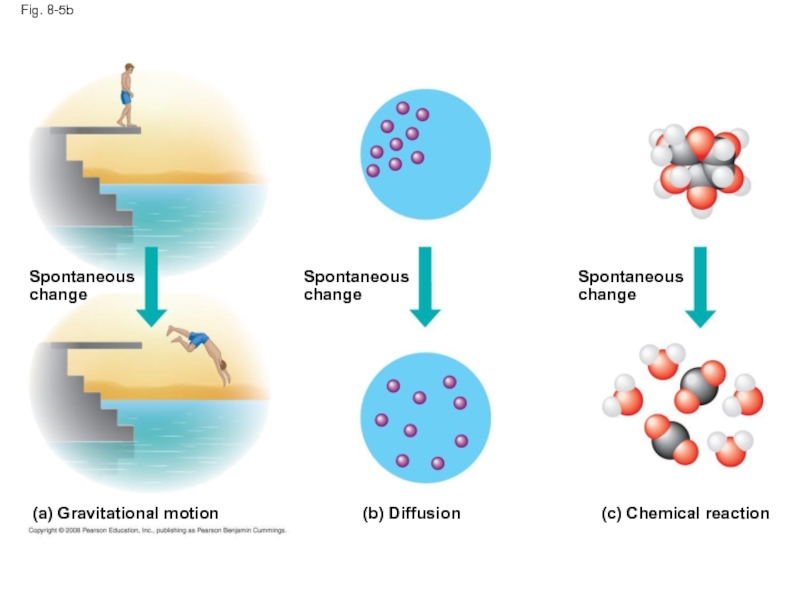
Слайд 26Fig. 8-5b
Spontaneous
change
Spontaneous
change
Spontaneous
change
(b) Diffusion
(c) Chemical reaction
(a) Gravitational motion
Слайд 27Free Energy and Metabolism
The concept of free energy can be applied
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
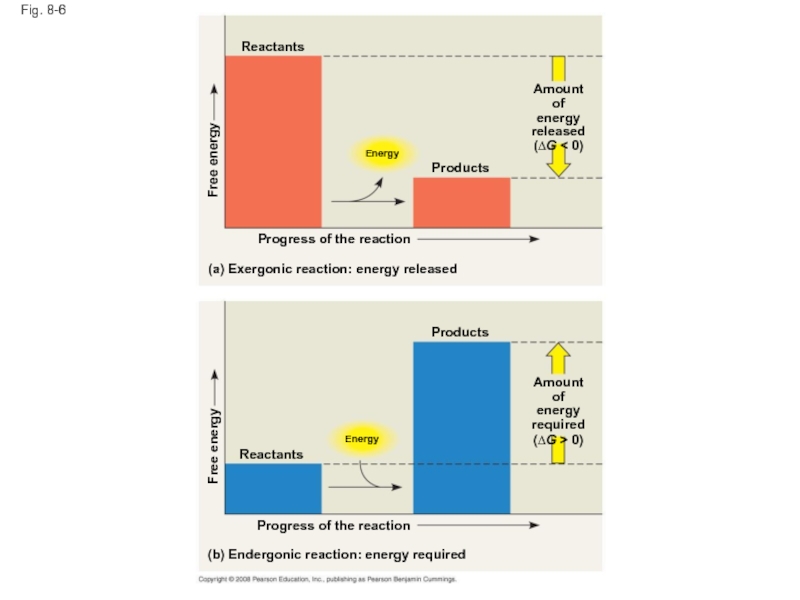
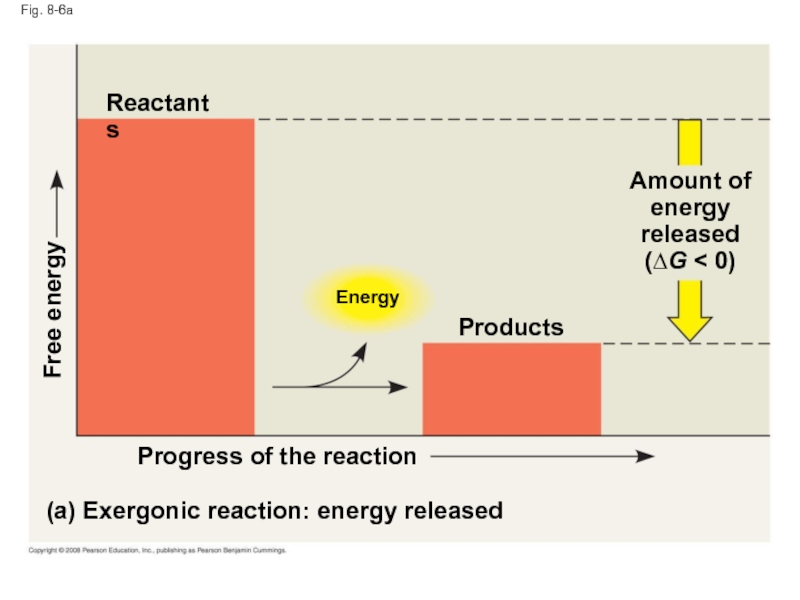
Слайд 28Exergonic and Endergonic Reactions in Metabolism
An exergonic reaction proceeds with a
An endergonic reaction absorbs free energy from its surroundings and is nonspontaneous
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 29Fig. 8-6
Reactants
Energy
Free energy
Products
Amount of
energy
released
(∆G < 0)
Progress of the reaction
(a) Exergonic reaction:
Products
Reactants
Energy
Free energy
Amount of
energy
required
(∆G > 0)
(b) Endergonic reaction: energy required
Progress of the reaction
Слайд 30Fig. 8-6a
Energy
(a) Exergonic reaction: energy released
Progress of the reaction
Free energy
Products
Amount of
energy
released
(∆G
Reactants
Слайд 31Fig. 8-6b
Energy
(b) Endergonic reaction: energy required
Progress of the reaction
Free energy
Products
Amount of
energy
required
(∆G
Reactants
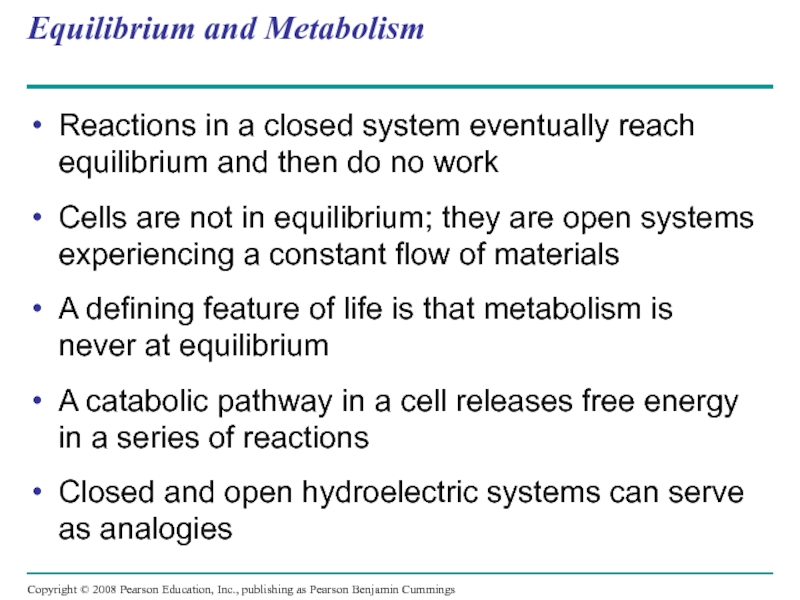
Слайд 32Equilibrium and Metabolism
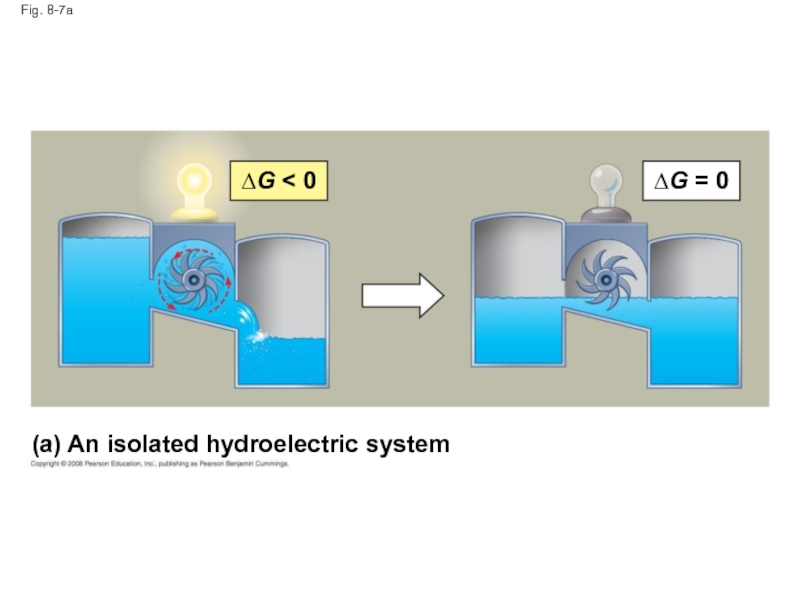
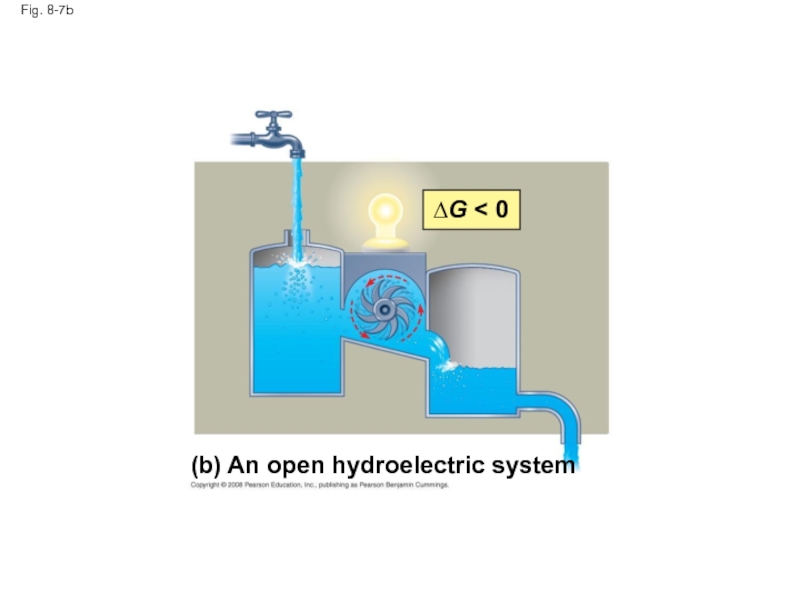
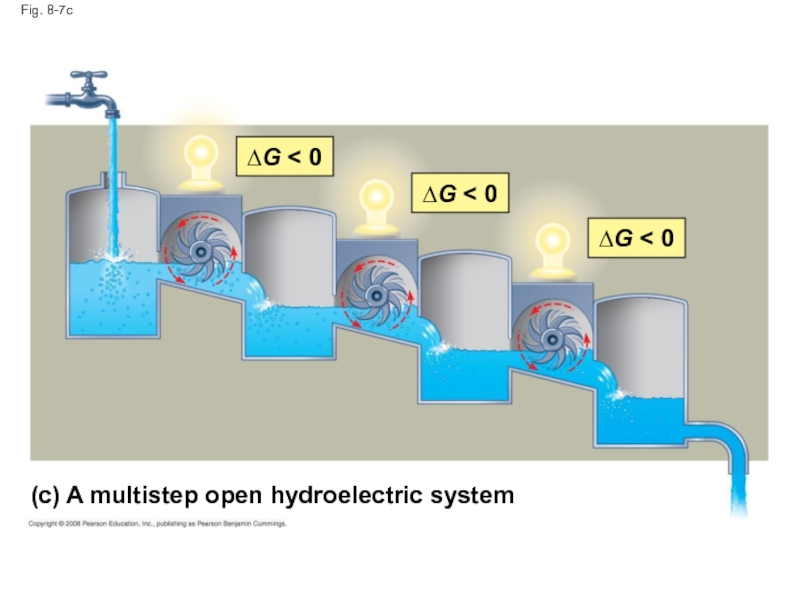
Reactions in a closed system eventually reach equilibrium and
Cells are not in equilibrium; they are open systems experiencing a constant flow of materials
A defining feature of life is that metabolism is never at equilibrium
A catabolic pathway in a cell releases free energy in a series of reactions
Closed and open hydroelectric systems can serve as analogies
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 33Fig. 8-7
(a) An isolated hydroelectric system
∆G < 0
∆G = 0
(b) An
system
∆G < 0
∆G < 0
∆G < 0
∆G < 0
(c) A multistep open hydroelectric system
Слайд 37Concept 8.3: ATP powers cellular work by coupling exergonic reactions to
A cell does three main kinds of work:
Chemical
Transport
Mechanical
To do work, cells manage energy resources by energy coupling, the use of an exergonic process to drive an endergonic one
Most energy coupling in cells is mediated by ATP
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
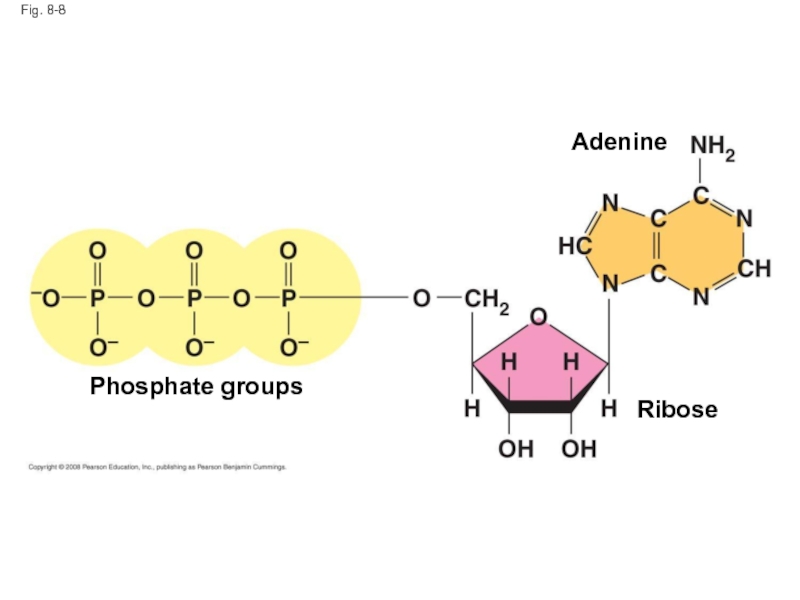
Слайд 38The Structure and Hydrolysis of ATP
ATP (adenosine triphosphate) is the cell’s
ATP is composed of ribose (a sugar), adenine (a nitrogenous base), and three phosphate groups
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
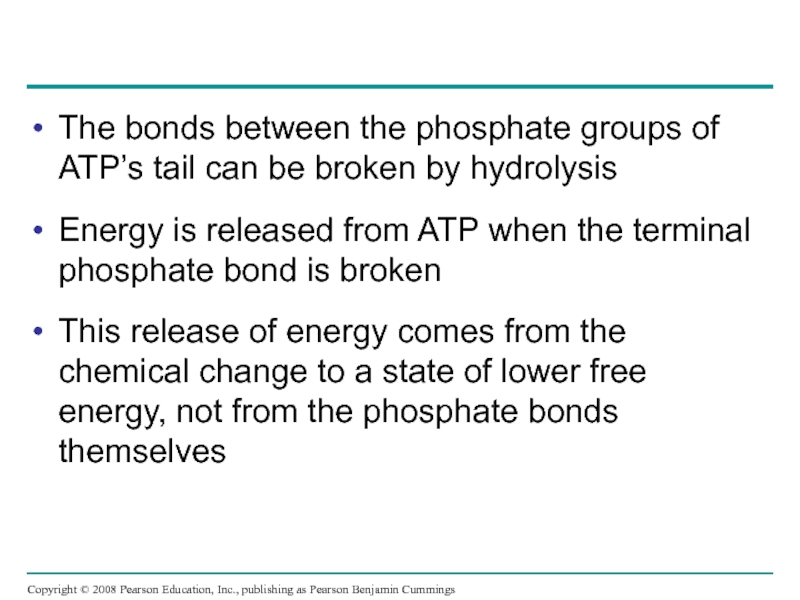
Слайд 40The bonds between the phosphate groups of ATP’s tail can be
Energy is released from ATP when the terminal phosphate bond is broken
This release of energy comes from the chemical change to a state of lower free energy, not from the phosphate bonds themselves
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
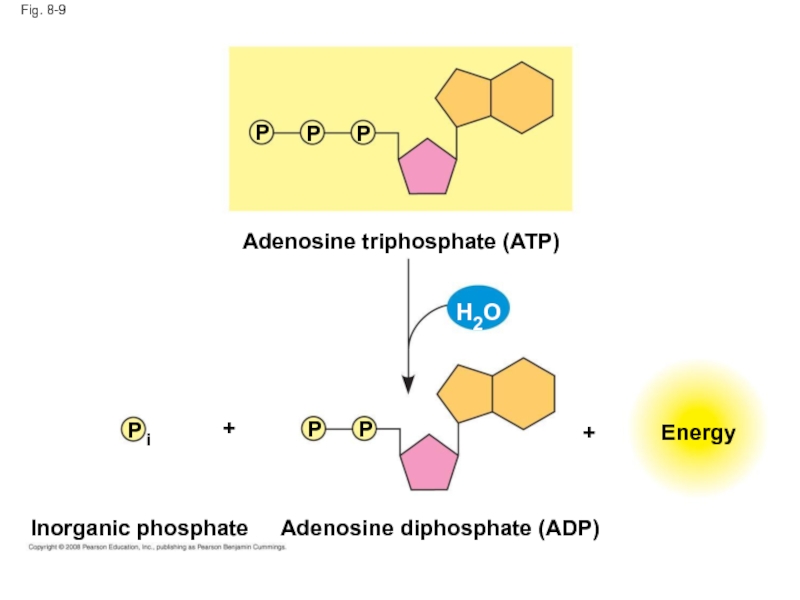
Слайд 41Fig. 8-9
Inorganic phosphate
Energy
Adenosine triphosphate (ATP)
Adenosine diphosphate (ADP)
P
P
P
P
P
P
+
+
H2O
i
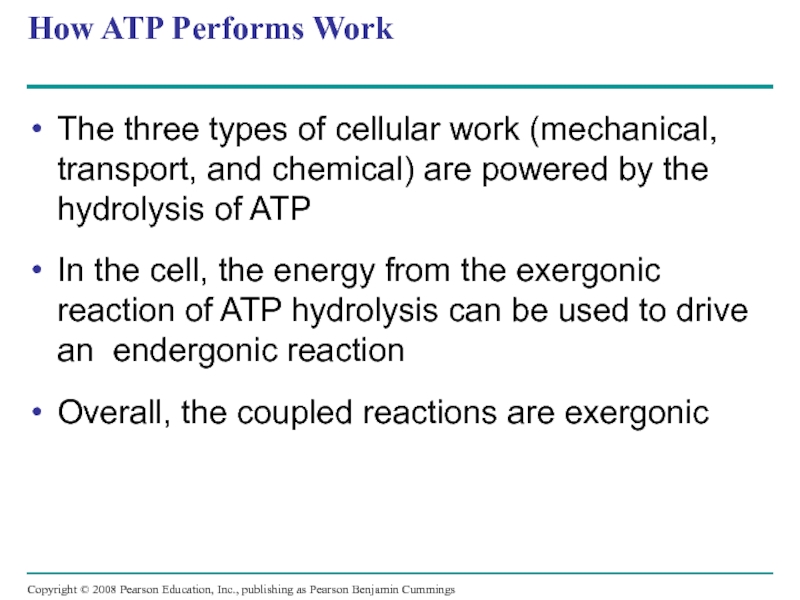
Слайд 42How ATP Performs Work
The three types of cellular work (mechanical, transport,
In the cell, the energy from the exergonic reaction of ATP hydrolysis can be used to drive an endergonic reaction
Overall, the coupled reactions are exergonic
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
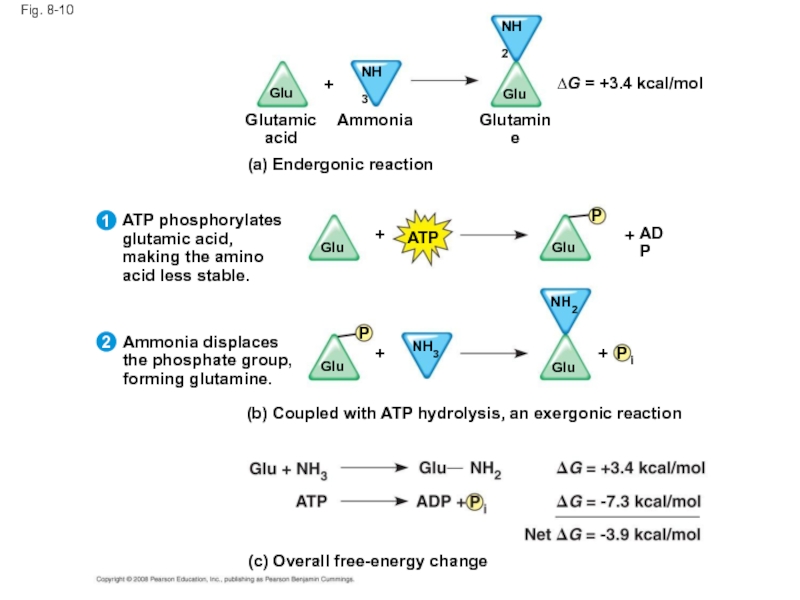
Слайд 43Fig. 8-10
(b) Coupled with ATP hydrolysis, an exergonic reaction
Ammonia displaces
the phosphate
forming glutamine.
(a) Endergonic reaction
(c) Overall free-energy change
P
P
Glu
NH3
NH2
Glu
i
Glu
ADP
+
P
ATP
+
+
Glu
ATP phosphorylates
glutamic acid,
making the amino
acid less stable.
Glu
NH3
NH2
Glu
+
Glutamic
acid
Glutamine
Ammonia
∆G = +3.4 kcal/mol
+
2
1
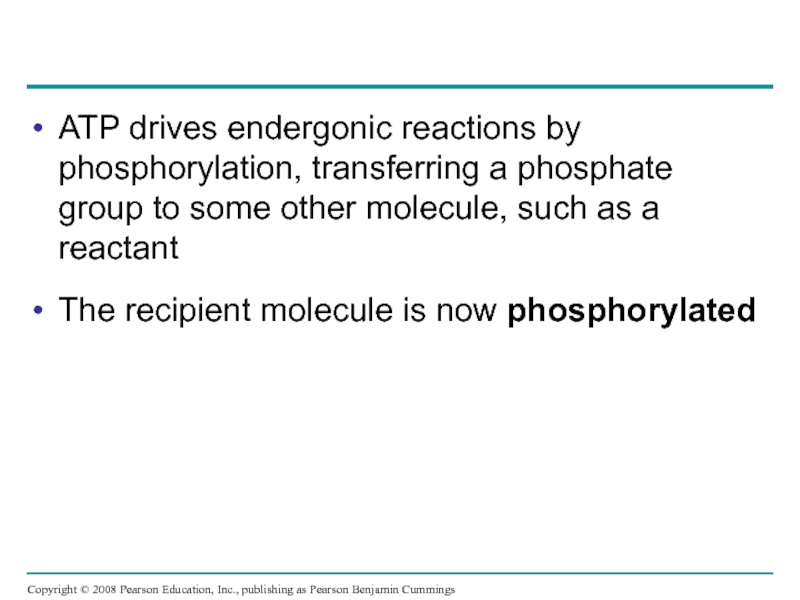
Слайд 44ATP drives endergonic reactions by phosphorylation, transferring a phosphate group to
The recipient molecule is now phosphorylated
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
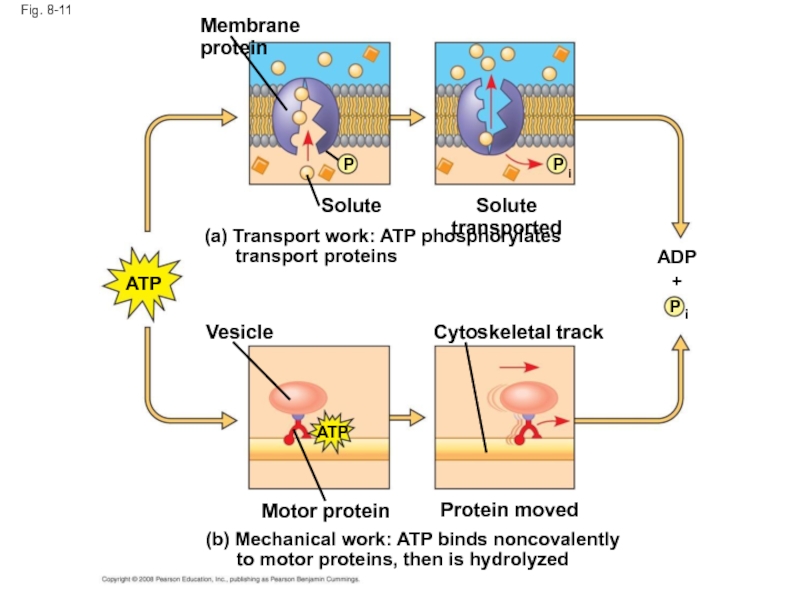
Слайд 45Fig. 8-11
(b) Mechanical work: ATP binds noncovalently
to motor
Membrane protein
P
i
ADP
+
P
Solute
Solute transported
P
i
Vesicle
Cytoskeletal track
Motor protein
Protein moved
(a) Transport work: ATP phosphorylates
transport proteins
ATP
ATP
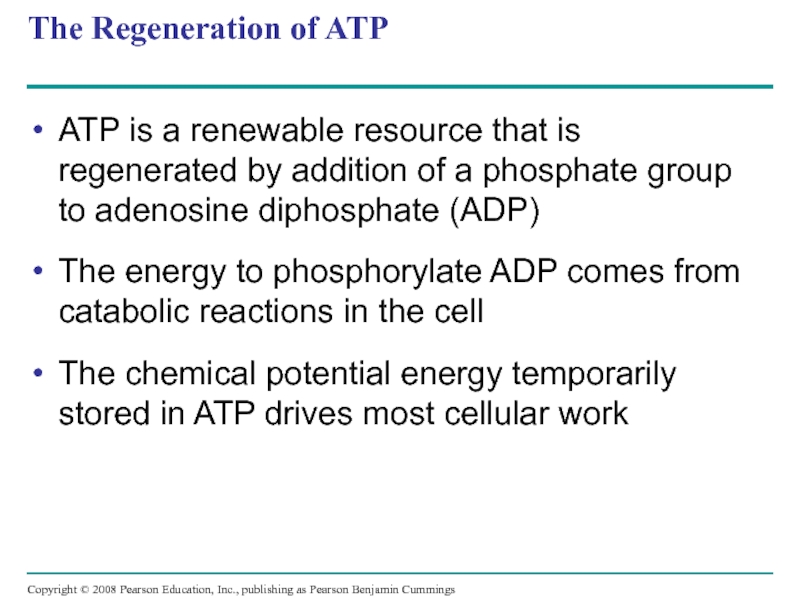
Слайд 46The Regeneration of ATP
ATP is a renewable resource that is regenerated
The energy to phosphorylate ADP comes from catabolic reactions in the cell
The chemical potential energy temporarily stored in ATP drives most cellular work
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 47Fig. 8-12
P
i
ADP
+
Energy from
catabolism (exergonic,
energy-releasing
processes)
Energy for cellular
work (endergonic,
energy-consuming
processes)
ATP
+
H2O
Слайд 48Concept 8.4: Enzymes speed up metabolic reactions by lowering energy barriers
A
An enzyme is a catalytic protein
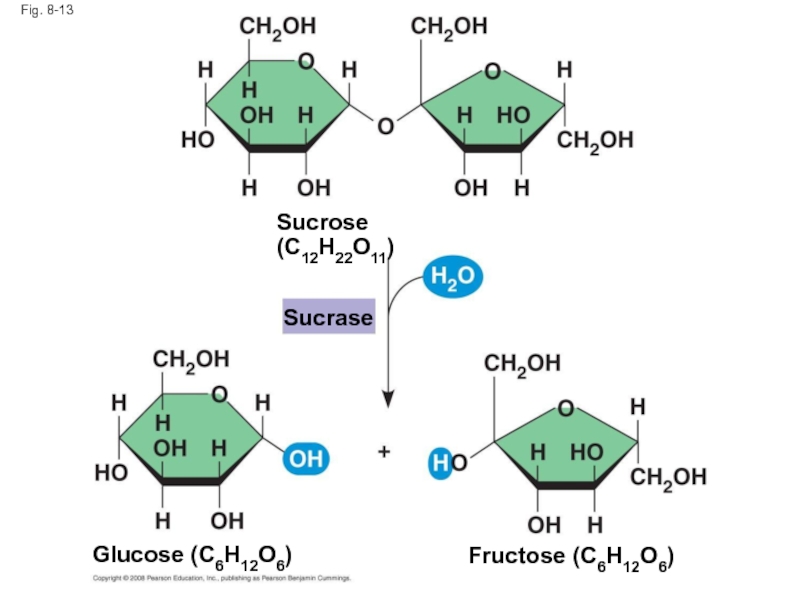
Hydrolysis of sucrose by the enzyme sucrase is an example of an enzyme-catalyzed reaction
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
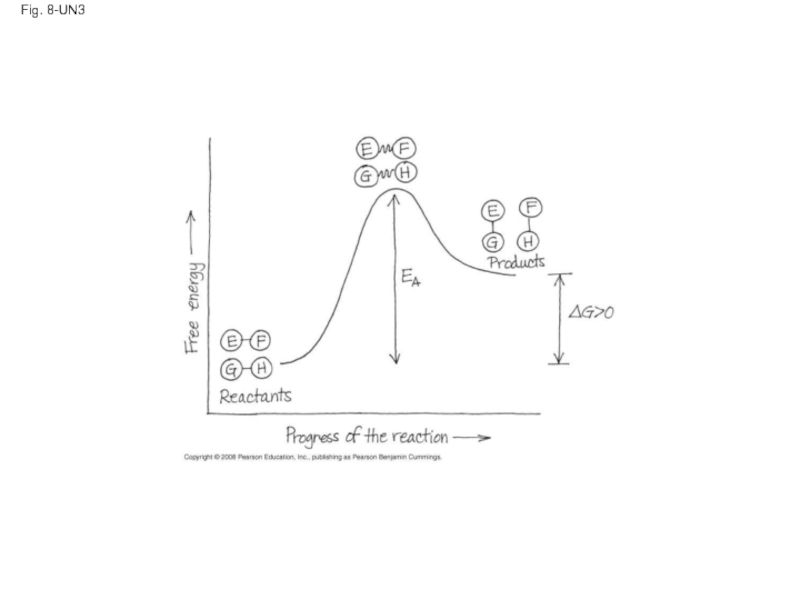
Слайд 50The Activation Energy Barrier
Every chemical reaction between molecules involves bond breaking
The initial energy needed to start a chemical reaction is called the free energy of activation, or activation energy (EA)
Activation energy is often supplied in the form of heat from the surroundings
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
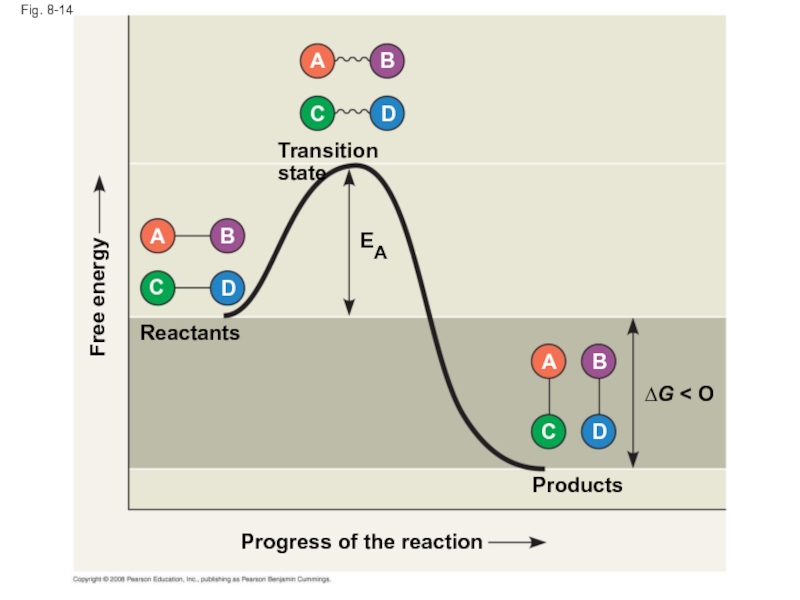
Слайд 51Fig. 8-14
Progress of the reaction
Products
Reactants
∆G < O
Transition state
Free energy
EA
D
C
B
A
D
D
C
C
B
B
A
A
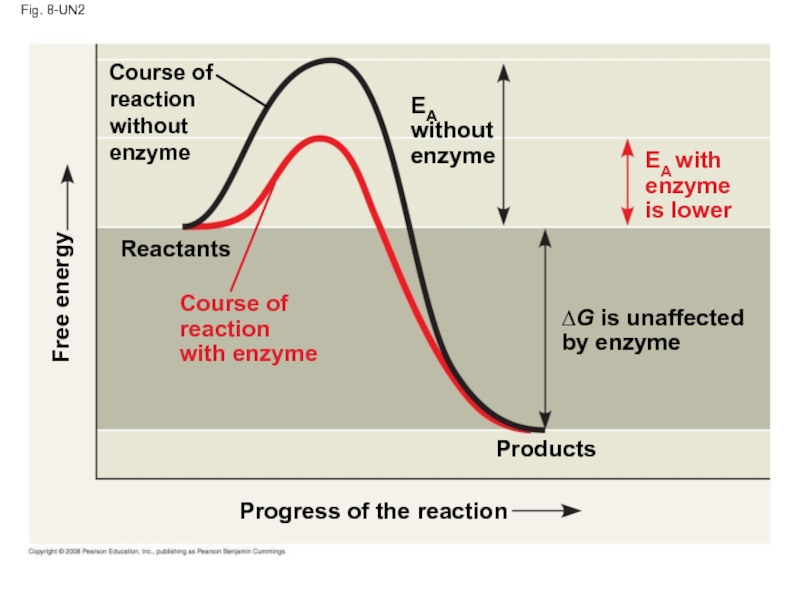
Слайд 52How Enzymes Lower the EA Barrier
Enzymes catalyze reactions by lowering the
Enzymes do not affect the change in free energy (∆G); instead, they hasten reactions that would occur eventually
Animation: How Enzymes Work
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
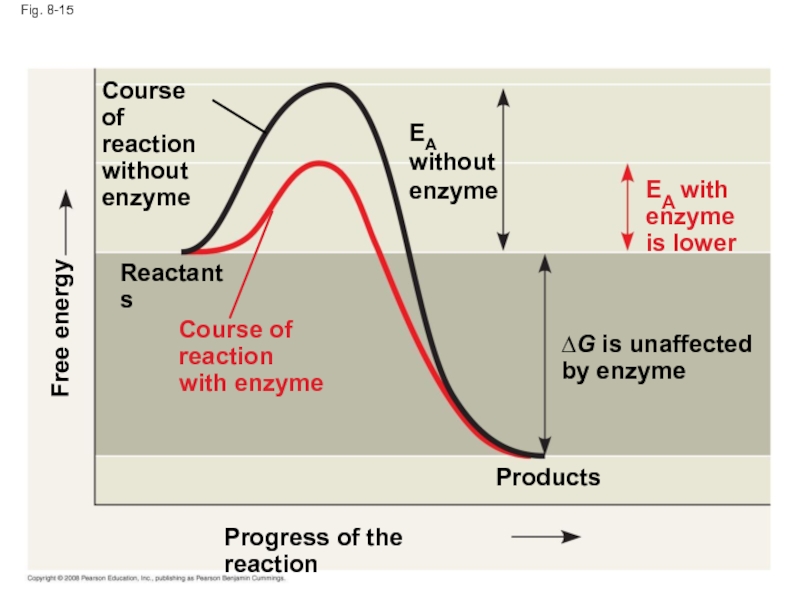
Слайд 53Fig. 8-15
Progress of the reaction
Products
Reactants
∆G is unaffected
by enzyme
Course of
reaction
without
enzyme
Free energy
EA
without
enzyme
EA with
enzyme
is
Course of
reaction
with enzyme
Слайд 54Substrate Specificity of Enzymes
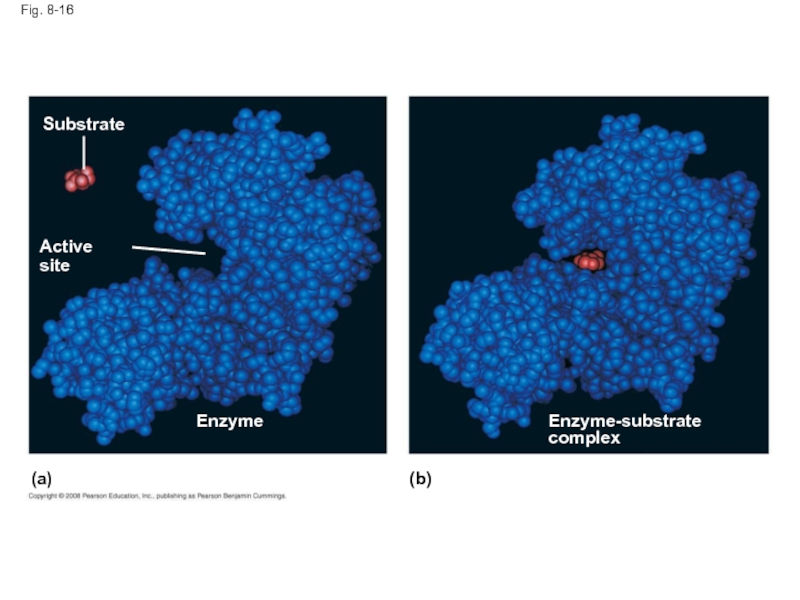
The reactant that an enzyme acts on is
The enzyme binds to its substrate, forming an enzyme-substrate complex
The active site is the region on the enzyme where the substrate binds
Induced fit of a substrate brings chemical groups of the active site into positions that enhance their ability to catalyze the reaction
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
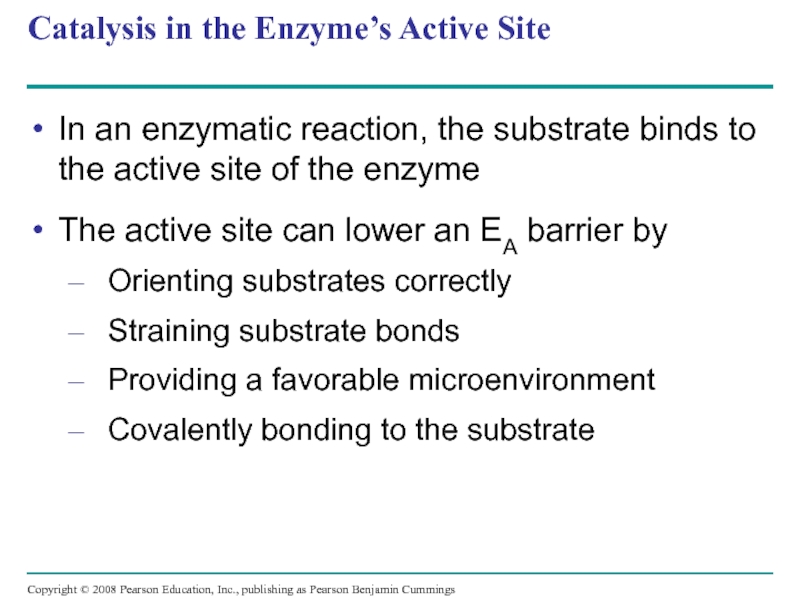
Слайд 56Catalysis in the Enzyme’s Active Site
In an enzymatic reaction, the substrate
The active site can lower an EA barrier by
Orienting substrates correctly
Straining substrate bonds
Providing a favorable microenvironment
Covalently bonding to the substrate
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
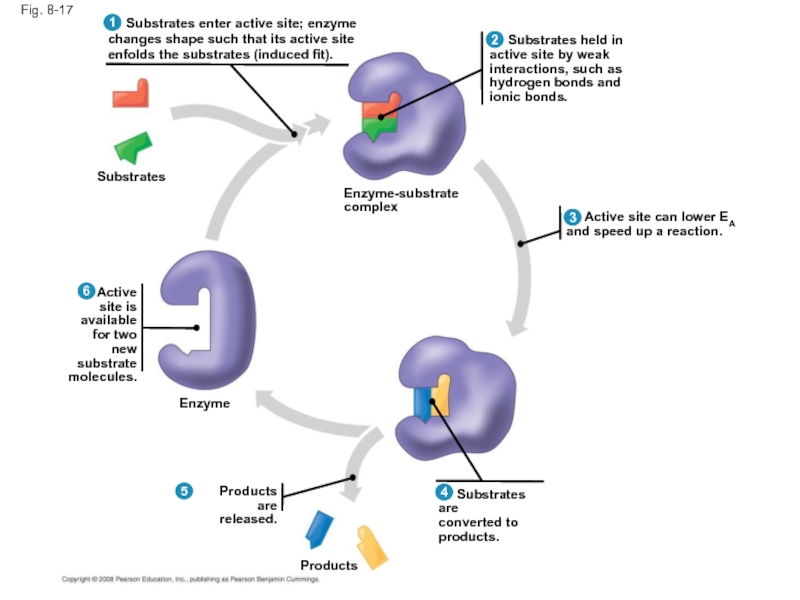
Слайд 57Fig. 8-17
Substrates
Enzyme
Products are
released.
Products
Substrates are
converted to
products.
Active
and speed up a reaction.
Substrates held in
active site by weak
interactions, such as
hydrogen bonds and
ionic bonds.
Substrates enter active site; enzyme
changes shape such that its active site
enfolds the substrates (induced fit).
Active
site is
available
for two new
substrate
molecules.
Enzyme-substrate
complex
5
3
2
1
6
4
Слайд 58Effects of Local Conditions on Enzyme Activity
An enzyme’s activity can be
General environmental factors, such as temperature and pH
Chemicals that specifically influence the enzyme
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 59Effects of Temperature and pH
Each enzyme has an optimal temperature in
Each enzyme has an optimal pH in which it can function
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 60Fig. 8-18
Rate of reaction
Optimal temperature for
enzyme of thermophilic
(heat-tolerant)
bacteria
Optimal temperature for
typical human enzyme
(a) Optimal temperature for two enzymes
(b) Optimal pH for two enzymes
Rate of reaction
Optimal pH for pepsin
(stomach enzyme)
Optimal pH
for trypsin
(intestinal
enzyme)
Temperature (ºC)
pH
5
4
3
2
1
0
6
7
8
9
10
0
20
40
80
60
100
Слайд 61Cofactors
Cofactors are nonprotein enzyme helpers
Cofactors may be inorganic (such as a
An organic cofactor is called a coenzyme
Coenzymes include vitamins
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
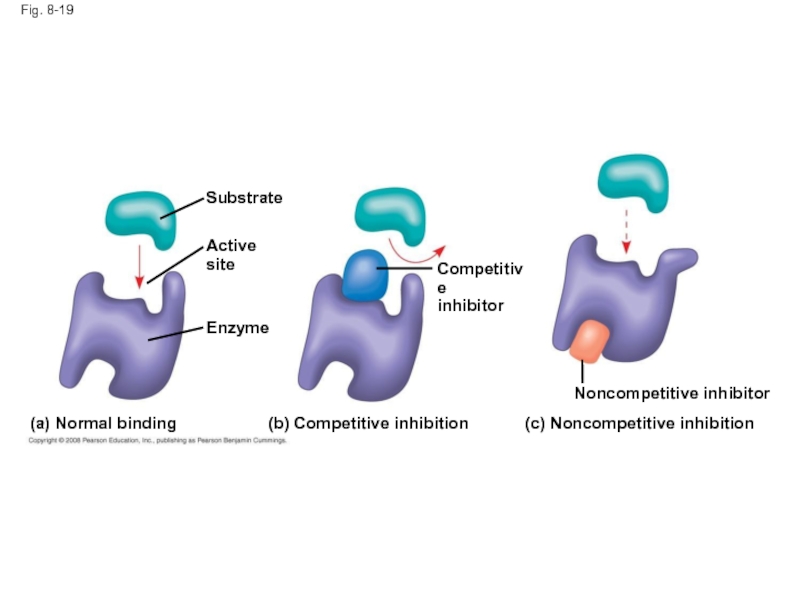
Слайд 62Enzyme Inhibitors
Competitive inhibitors bind to the active site of an enzyme,
Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective
Examples of inhibitors include toxins, poisons, pesticides, and antibiotics
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 63Fig. 8-19
(a) Normal binding
(c) Noncompetitive inhibition
(b) Competitive inhibition
Noncompetitive inhibitor
Active site
Competitive
inhibitor
Substrate
Enzyme
Слайд 64Concept 8.5: Regulation of enzyme activity helps control metabolism
Chemical chaos would
A cell does this by switching on or off the genes that encode specific enzymes or by regulating the activity of enzymes
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 65Allosteric Regulation of Enzymes
Allosteric regulation may either inhibit or stimulate an
Allosteric regulation occurs when a regulatory molecule binds to a protein at one site and affects the protein’s function at another site
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 66Allosteric Activation and Inhibition
Most allosterically regulated enzymes are made from polypeptide
Each enzyme has active and inactive forms
The binding of an activator stabilizes the active form of the enzyme
The binding of an inhibitor stabilizes the inactive form of the enzyme
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
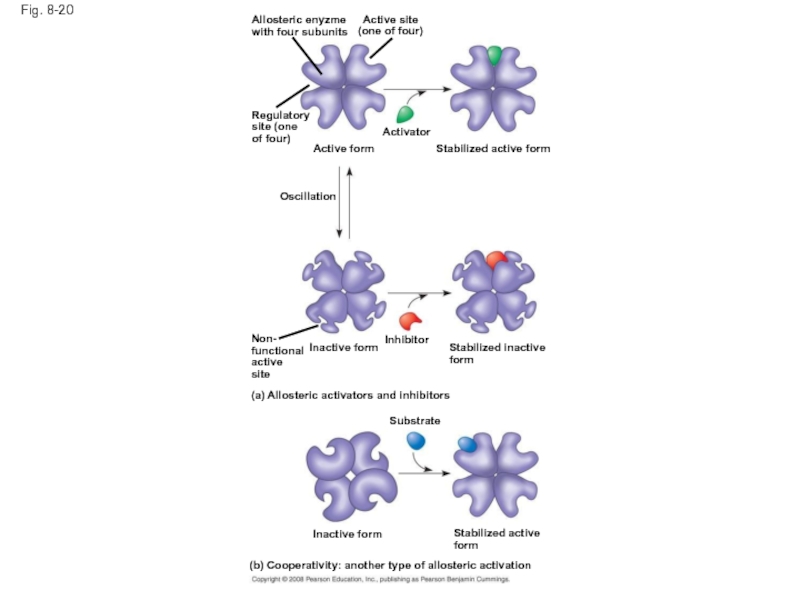
Слайд 67Fig. 8-20
Allosteric enyzme
with four subunits
Active site
(one of four)
Regulatory
site (one
of four)
Active form
Activator
Stabilized
Oscillation
Non-
functional
active
site
Inhibitor
Inactive form
Stabilized inactive
form
(a) Allosteric activators and inhibitors
Substrate
Inactive form
Stabilized active
form
(b) Cooperativity: another type of allosteric activation
Слайд 68Fig. 8-20a
(a) Allosteric activators and inhibitors
Inhibitor
Non-
functional
active
site
Stabilized inactive
form
Inactive form
Oscillation
Activator
Active form
Stabilized active form
Regulatory
site (one
of four)
Allosteric enzyme
with four subunits
Active site
(one of four)
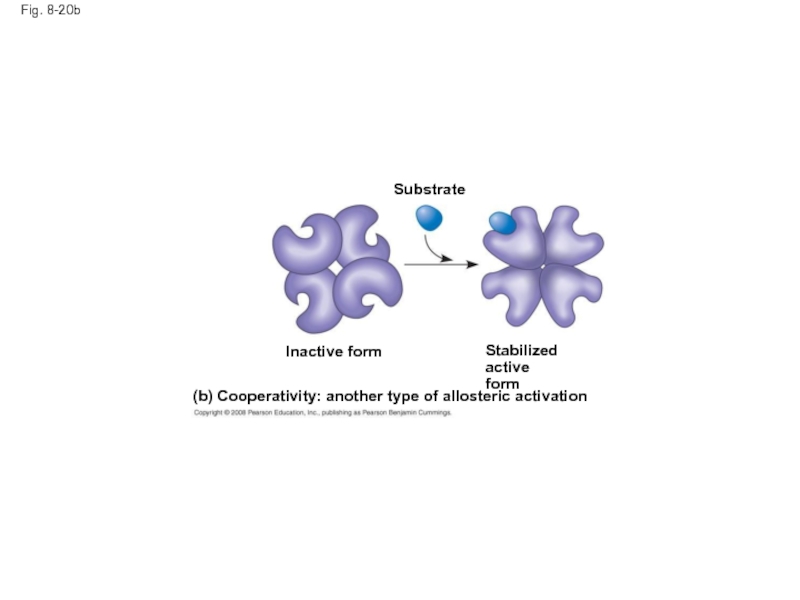
Слайд 69Cooperativity is a form of allosteric regulation that can amplify enzyme
In cooperativity, binding by a substrate to one active site stabilizes favorable conformational changes at all other subunits
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 70Fig. 8-20b
(b) Cooperativity: another type of allosteric activation
Stabilized active
form
Substrate
Inactive form
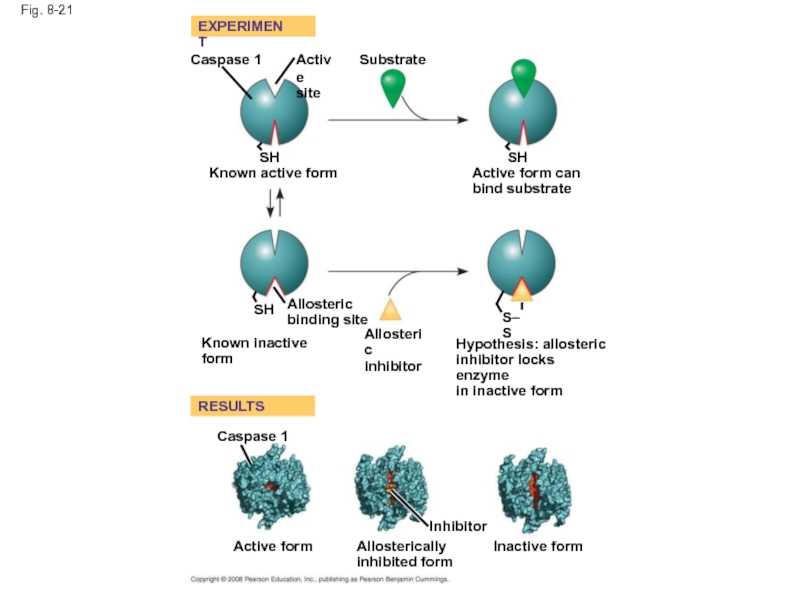
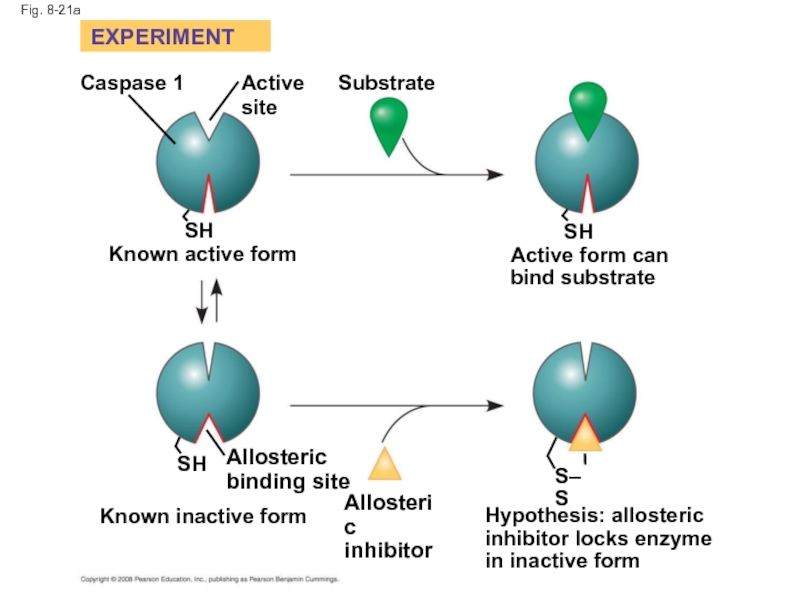
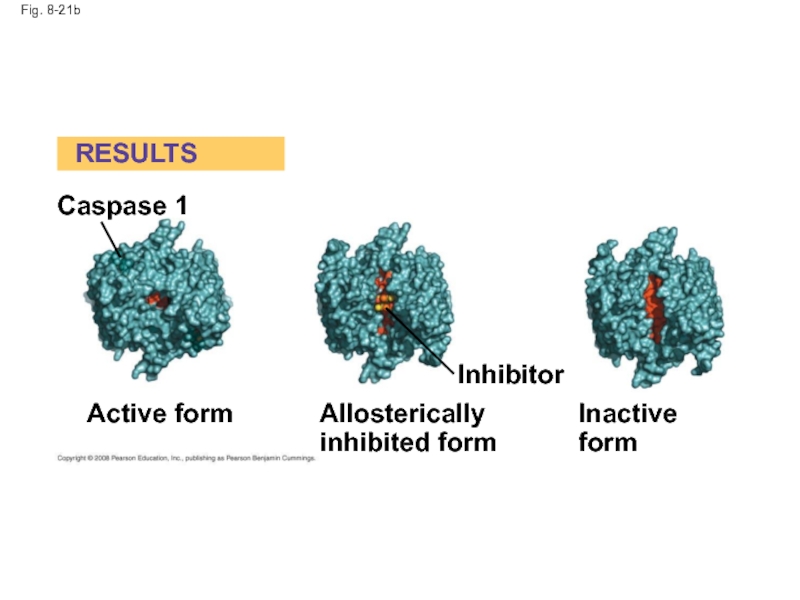
Слайд 71Identification of Allosteric Regulators
Allosteric regulators are attractive drug candidates for enzyme
Inhibition of proteolytic enzymes called caspases may help management of inappropriate inflammatory responses
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 72
Fig. 8-21
RESULTS
EXPERIMENT
Caspase 1
Active
site
SH
Known active form
Substrate
SH
Active form can
bind substrate
SH
Allosteric
binding site
Known inactive form
Allosteric
inhibitor
Hypothesis:
inhibitor locks enzyme
in inactive form
S–S
Caspase 1
Active form
Allosterically
inhibited form
Inhibitor
Inactive form
Слайд 73
Fig. 8-21a
SH
Substrate
Hypothesis: allosteric
inhibitor locks enzyme
in inactive form
Active form can
bind substrate
S–S
SH
SH
Active
site
Caspase 1
Known
Known inactive form
Allosteric
binding site
Allosteric
inhibitor
EXPERIMENT
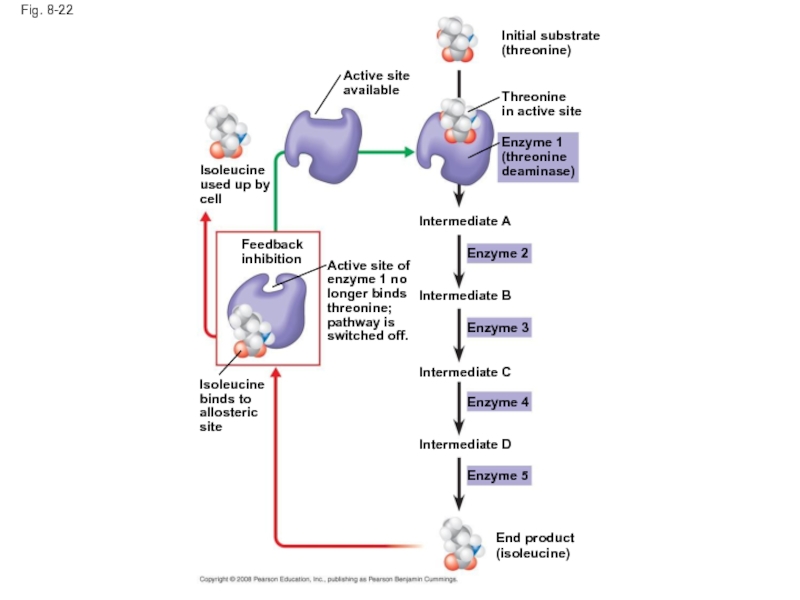
Слайд 75Feedback Inhibition
In feedback inhibition, the end product of a metabolic pathway
Feedback inhibition prevents a cell from wasting chemical resources by synthesizing more product than is needed
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 76Fig. 8-22
Intermediate C
Feedback
inhibition
Isoleucine
used up by
cell
Enzyme 1
(threonine
deaminase)
End product
(isoleucine)
Enzyme 5
Intermediate D
Intermediate B
Intermediate A
Enzyme
Enzyme 2
Enzyme 3
Initial substrate
(threonine)
Threonine
in active site
Active site
available
Active site of
enzyme 1 no
longer binds
threonine;
pathway is
switched off.
Isoleucine
binds to
allosteric
site
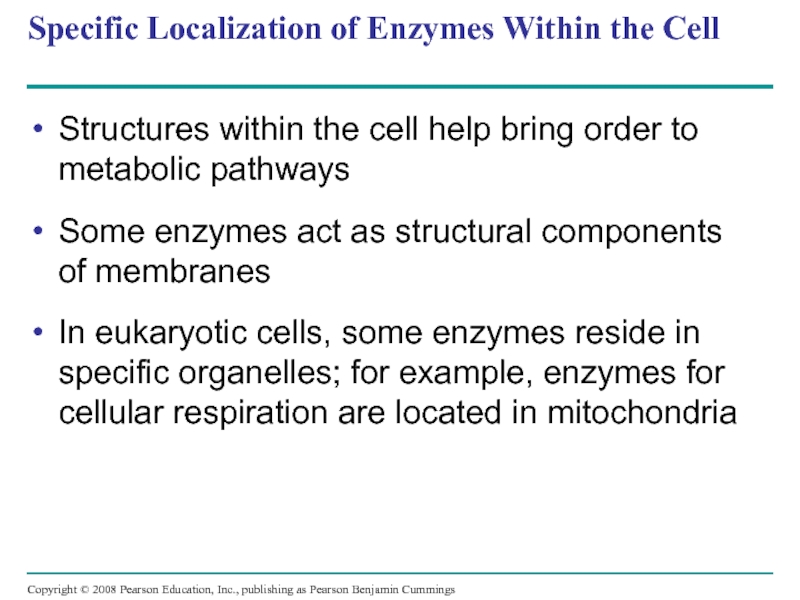
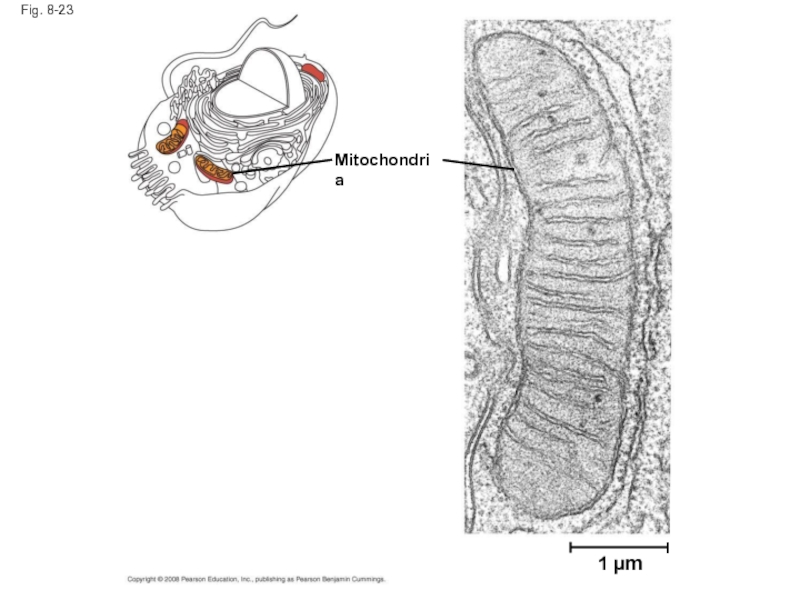
Слайд 77Specific Localization of Enzymes Within the Cell
Structures within the cell help
Some enzymes act as structural components of membranes
In eukaryotic cells, some enzymes reside in specific organelles; for example, enzymes for cellular respiration are located in mitochondria
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 79Fig. 8-UN2
Progress of the reaction
Products
Reactants
∆G is unaffected
by enzyme
Course of
reaction
without
enzyme
Free energy
EA
without
enzyme
EA with
enzyme
is
Course of
reaction
with enzyme
Слайд 83You should now be able to:
Distinguish between the following pairs of
In your own words, explain the second law of thermodynamics and explain why it is not violated by living organisms
Explain in general terms how cells obtain the energy to do cellular work
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
Слайд 84Explain how ATP performs cellular work
Explain why an investment of
Describe the mechanisms by which enzymes lower activation energy
Describe how allosteric regulators may inhibit or stimulate the activity of an enzyme
Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings