- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Accelerated aging diseases and their genetic causes Complex pattern of aging markers in primary human fibroblasts презентация
Содержание
- 1. Accelerated aging diseases and their genetic causes Complex pattern of aging markers in primary human fibroblasts
- 2. Primary non-transformed cell cultures tend to change
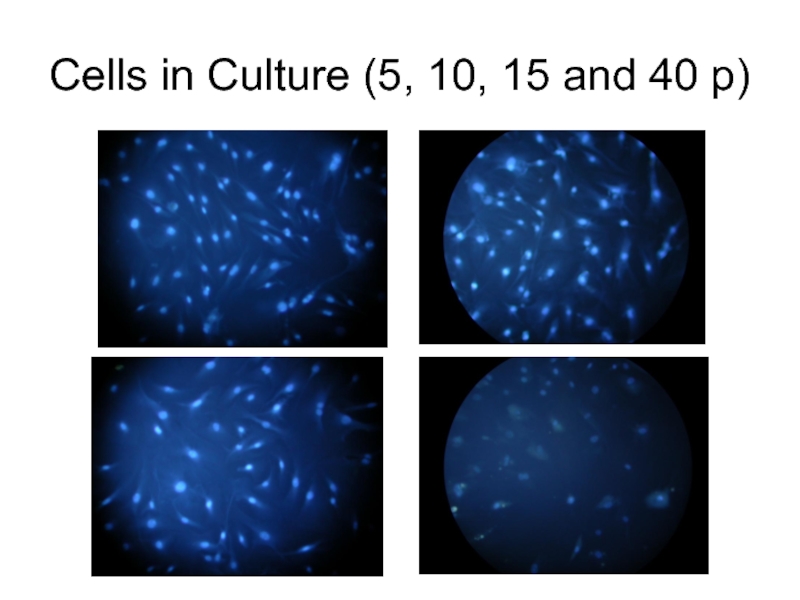
- 3. Cells in Culture (5, 10, 15 and 40 p)
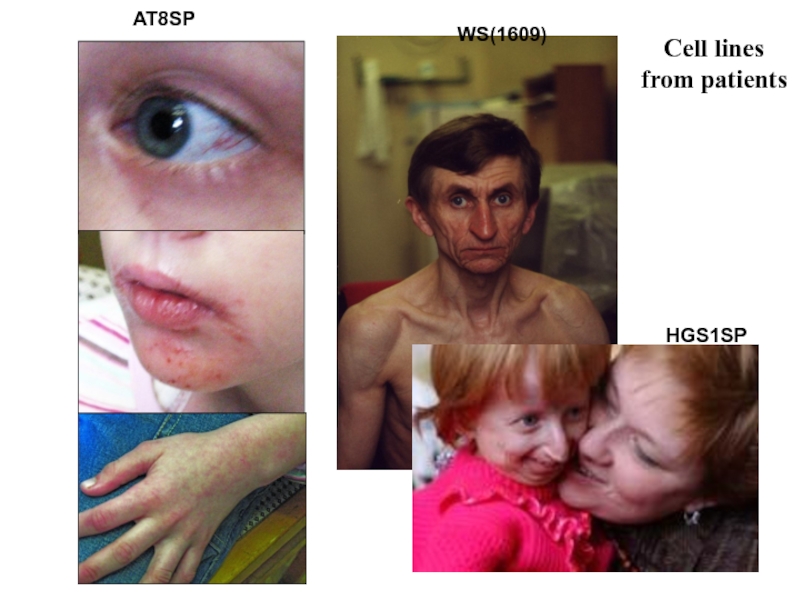
- 4. Cell lines from patients AT8SP HGS1SP WS(1609)
- 5. The most frequently used marker of aging
- 6. Primary fibroblasts of skin from donors of
- 7. Hutchinson-Gilford syndrome Autosomal dominant, emerging “de novo”,
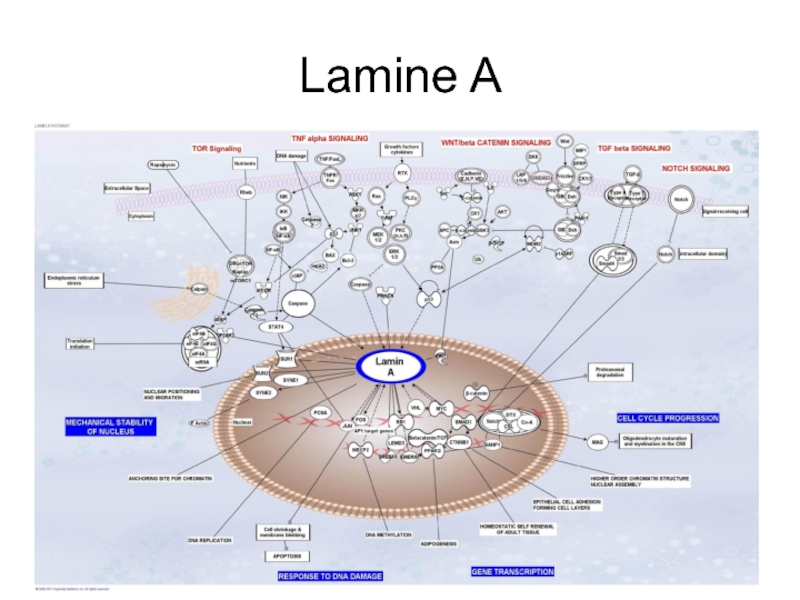
- 8. Lamine A
- 9. Nuclear lamina Structural transformations of the nuclear
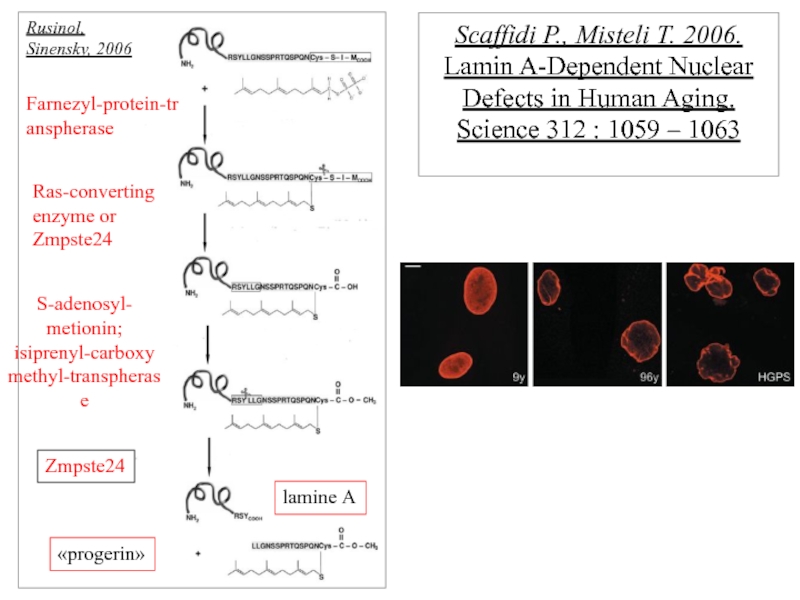
- 10. Scaffidi P., Misteli T. 2006. Lamin
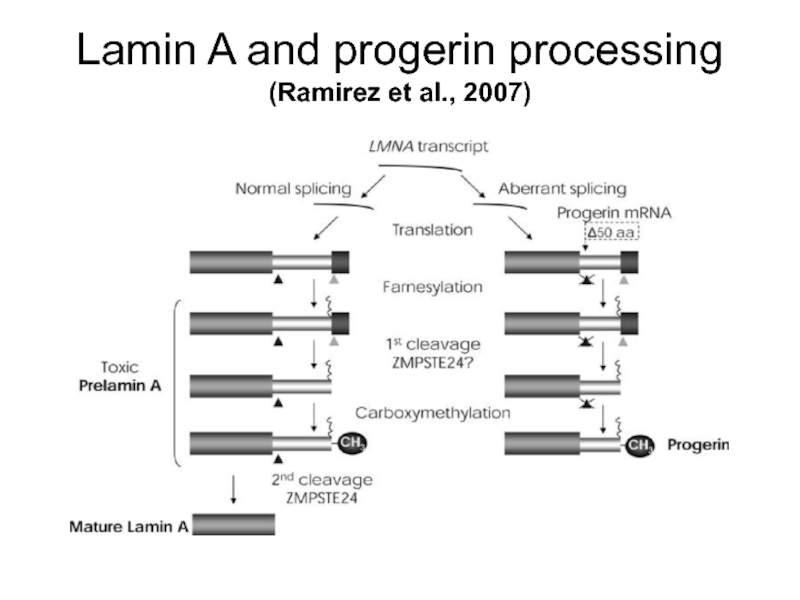
- 11. Lamin A and progerin processing (Ramirez et al., 2007)
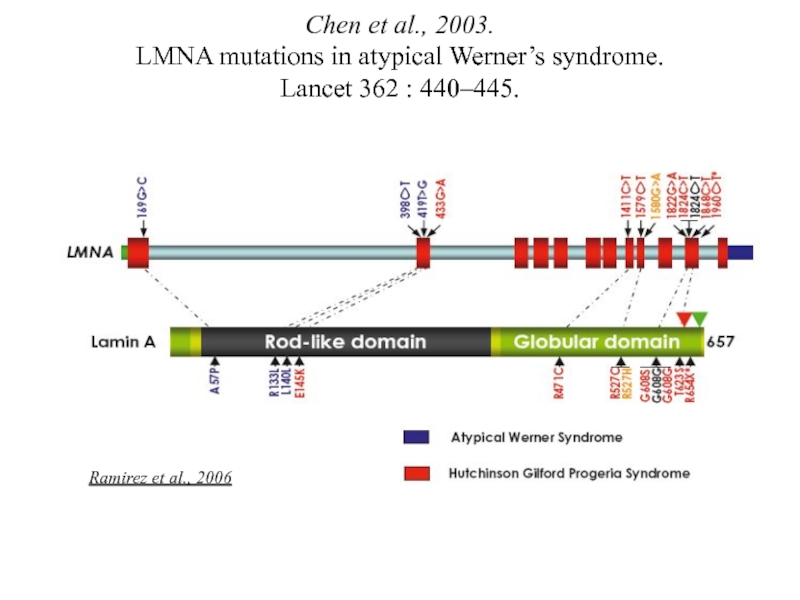
- 12. Chen et al., 2003. LMNA
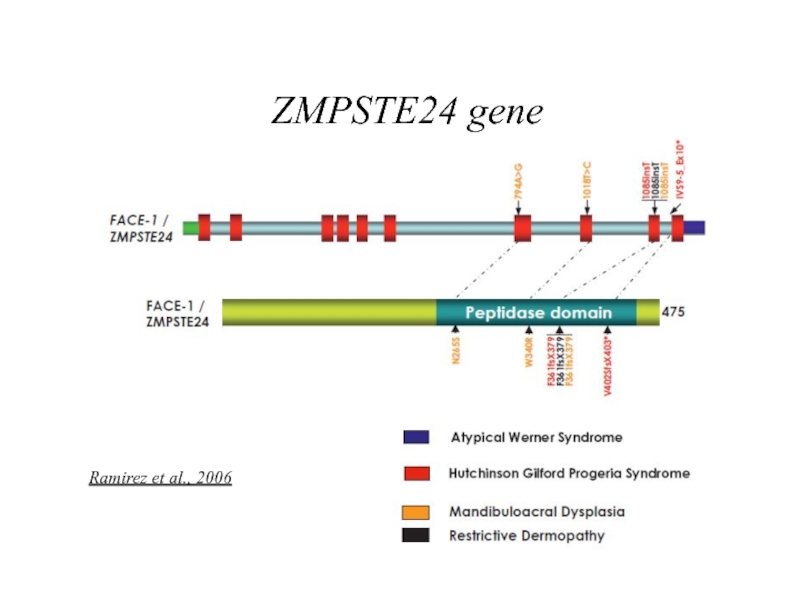
- 13. ZMPSTE24 gene Ramirez et al., 2006
- 14. Nuclear lamina (lamin A/C detection) The nuclear
- 15. Accumulation of foci of γ-Н2АХ is observed
- 16. Scaffidi P., Misteli T. 2006. Lamin A-dependent nuclear defects in human aging. Science. 312:1059-1063
- 17. Cells change their epigenetic status with age.
- 18. Scaffidi P., Misteli T. 2006. Lamin A-dependent nuclear defects in human aging. Science. 312:1059-1063
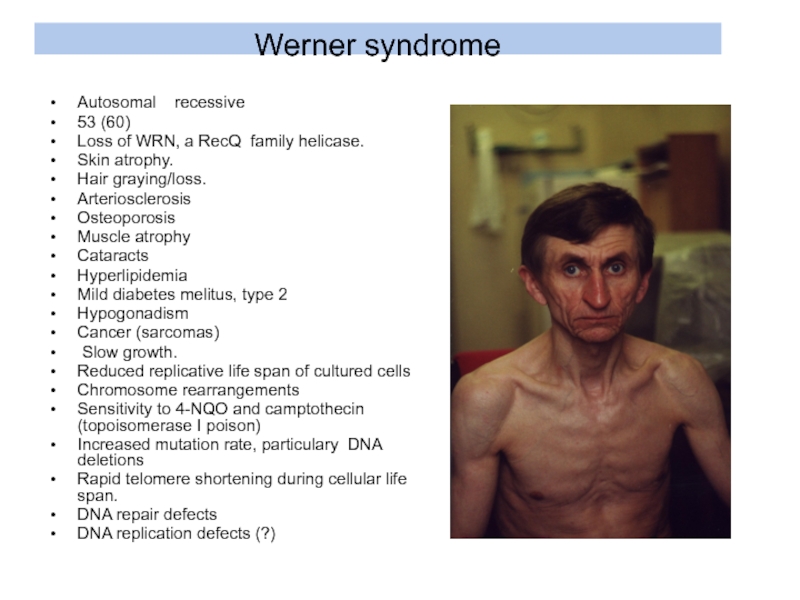
- 19. Werner syndrome Autosomal recessive 53
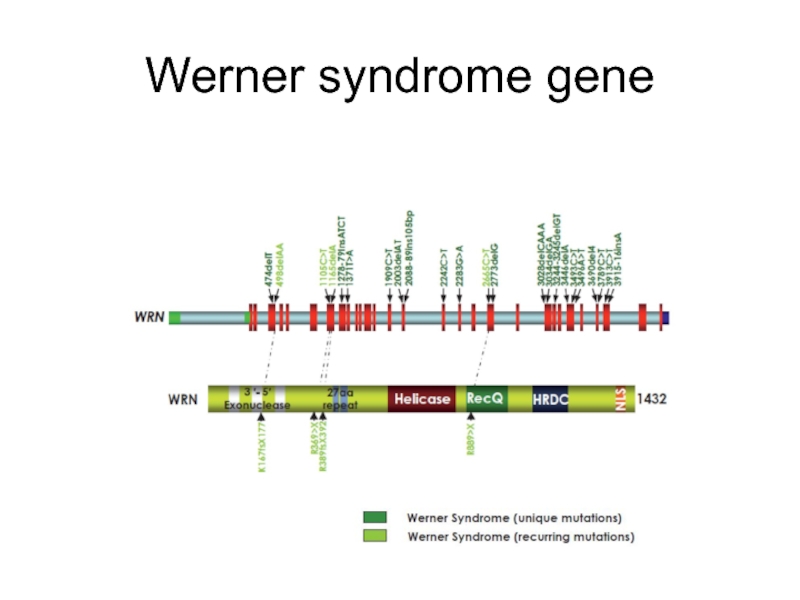
- 20. Werner syndrome gene
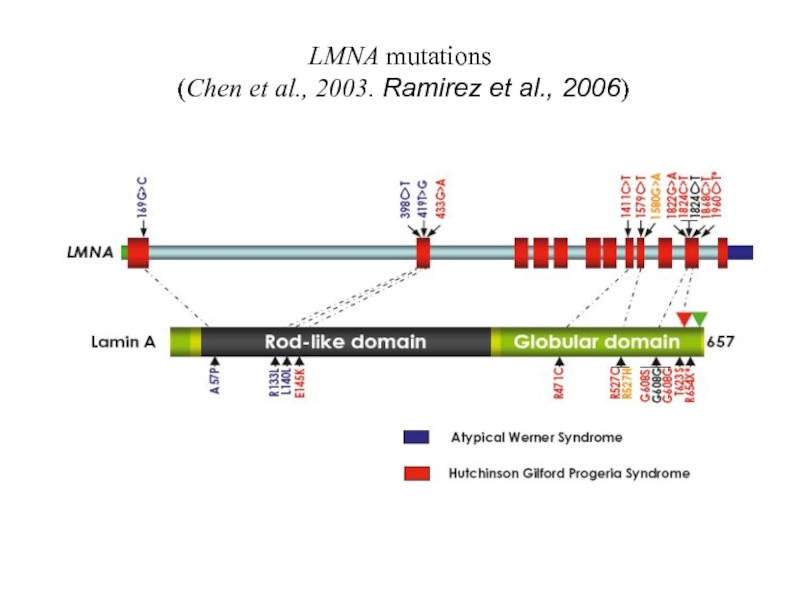
- 21. LMNA mutations (Chen et al., 2003. Ramirez et al., 2006)
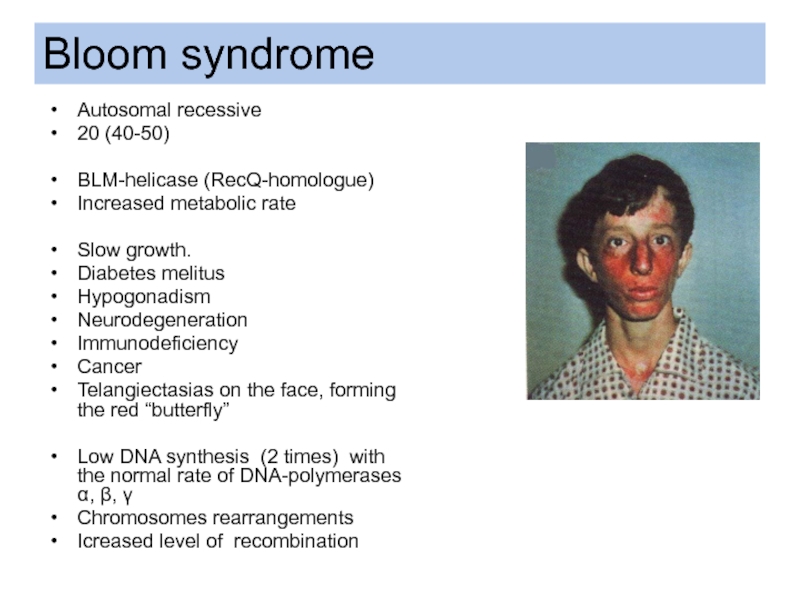
- 22. Autosomal recessive 20 (40-50) BLM-helicase (RecQ-homologue)
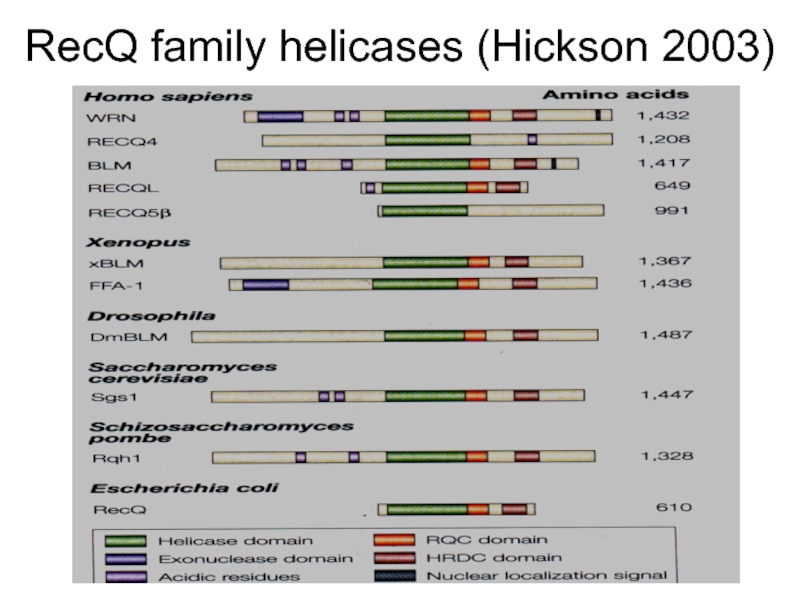
- 23. RecQ family helicases (Hickson 2003)
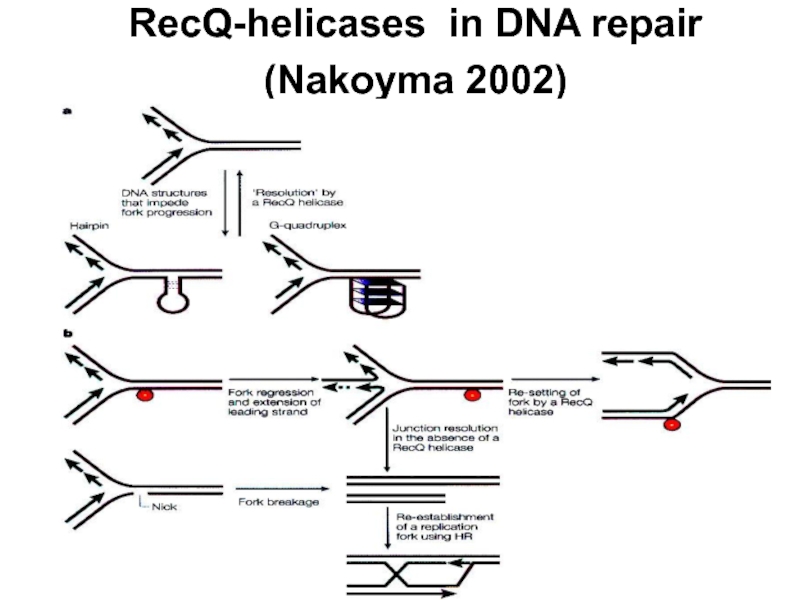
- 24. RecQ-helicases in DNA repair (Nakoyma 2002)
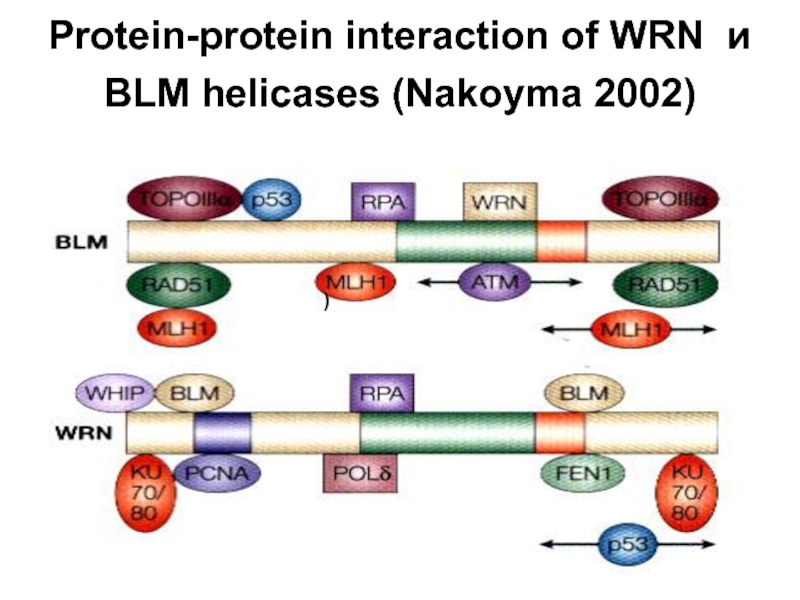
- 25. Protein-protein interaction of WRN и BLM helicases (Nakoyma 2002) )
- 26. In the study of cells from a
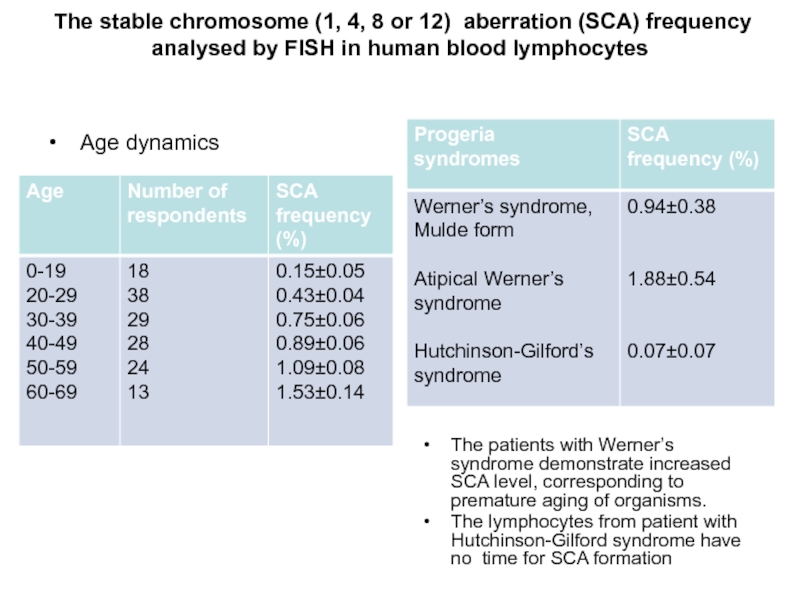
- 27. The stable chromosome (1, 4, 8
- 28. Autosomal recessive (40)-50 Skin atrophy DNA repair defects Cutix laxa
- 29. The telomere fragments lenght 1.HeLa
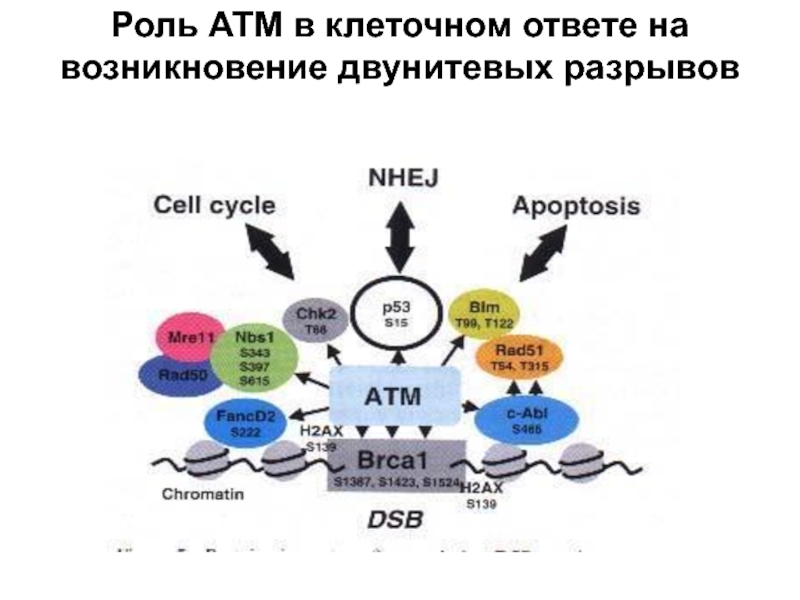
- 31. Роль АТМ в клеточном ответе на возникновение двунитевых разрывов
- 32. Autosomal recessive 20 (40-50) ATM, a protein
- 33. Атаксия-телеангиэктазия АТМ АТМ Дефекты репаративного синтеза
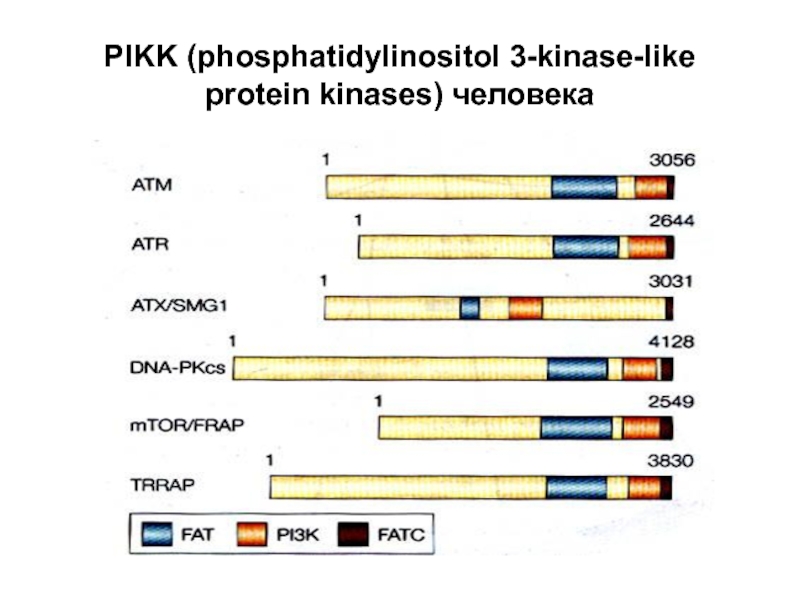
- 34. PIKK (phosphatidylinositol 3-kinase-like protein kinases) человека
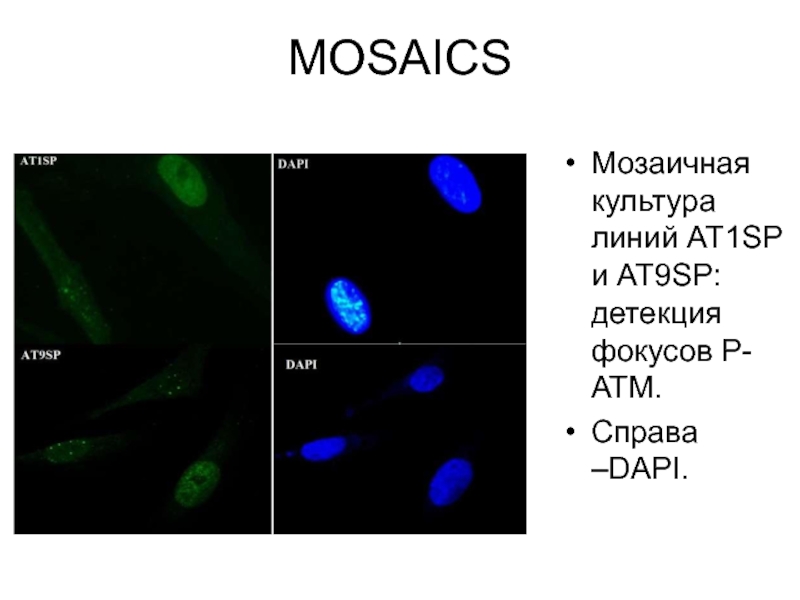
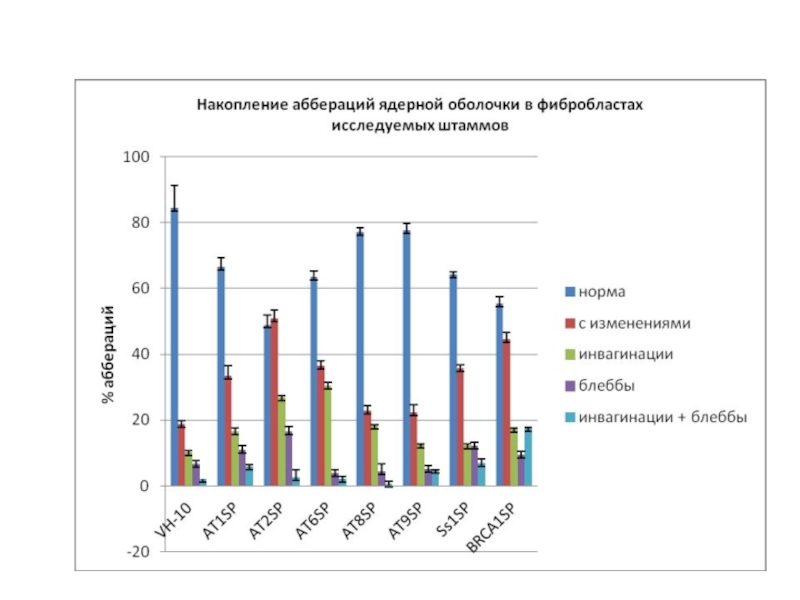
- 35. MOSAICS Мозаичная культура линий AT1SP и AT9SP: детекция фокусов Р-АТМ. Справа –DAPI.
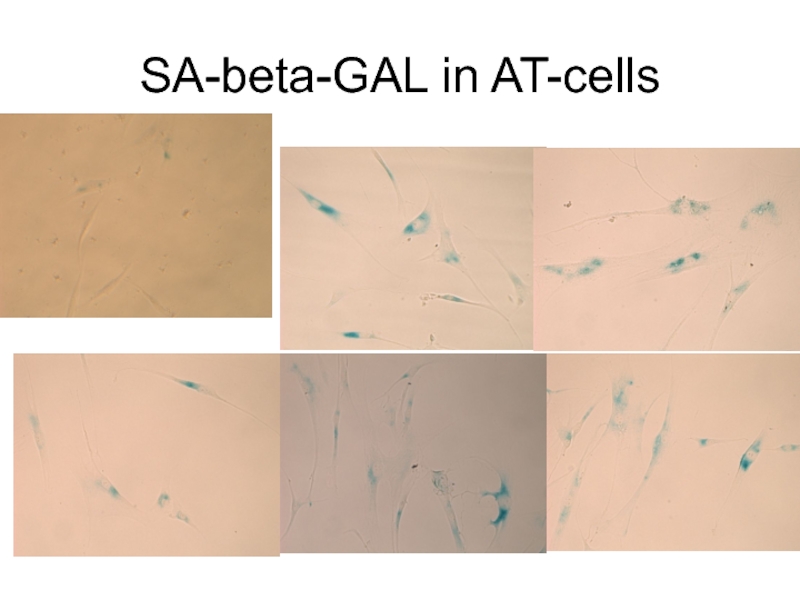
- 36. SA-beta-GAL in AT-cells
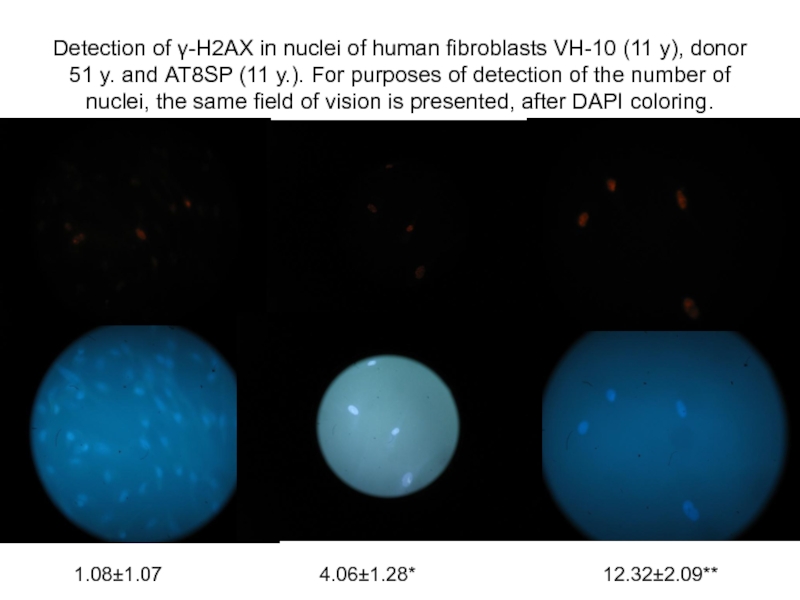
- 37. Detection of γ-Н2АХ in nuclei of human
- 40. DNA damage caused by ionizing radiation is
- 41. 53ВР1 foci in human fibroblast nuclei VH-10
- 42. Detection of НР1-γ in nuclei of human
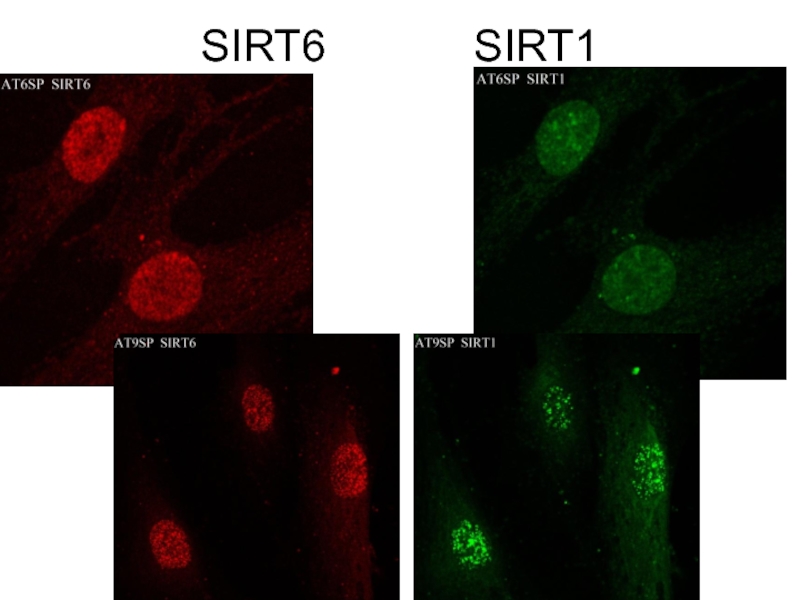
- 43. SIRT6 SIRT1
- 44. Detection of 3меК9Н3 in nuclei of human
- 45. 3meH3 fluorescence intensity in human fibroblasts In
- 46. Seckel syndrome (atr) O'Driscoll M, Ruiz-Perez VL,
- 47. Синдром Секкеля Микроцефалия, Умственная отсталость Карликовость Задержки
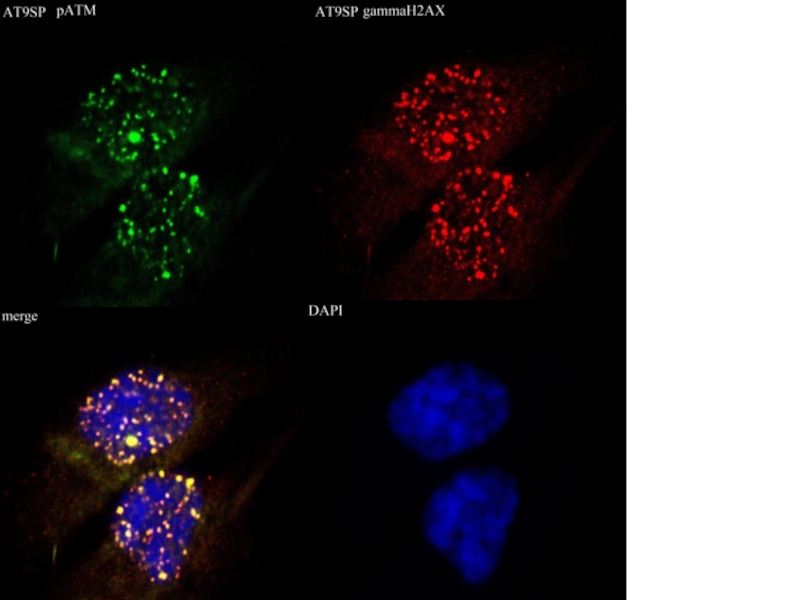
- 48. Совместная окраска антителами к рАТМ и активным
- 49. Ss1SP BRCA1SP
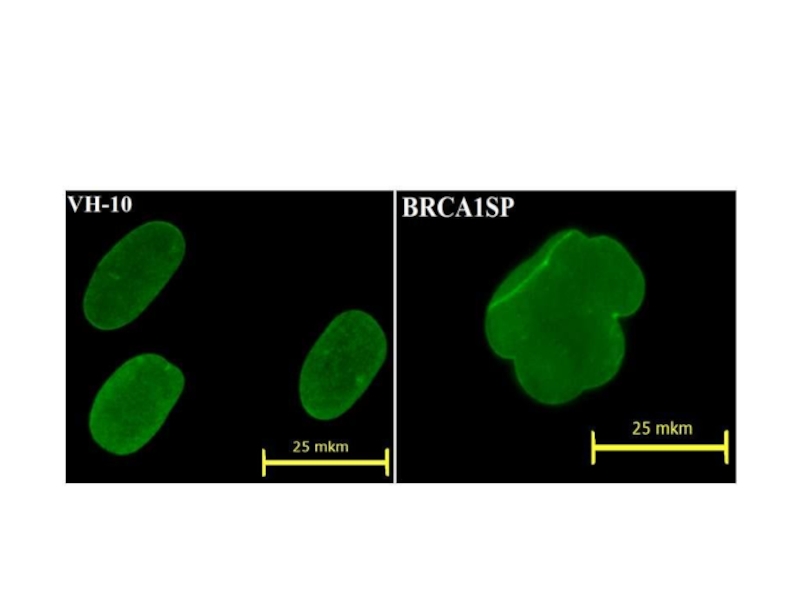
- 50. Ядерная оболочка, окраска антителами к ламину А\С
- 53. Mutations in TRAIP cause primordial dwarfism Harley
- 54. TRAIP localizes to sites of UV-induced DNA
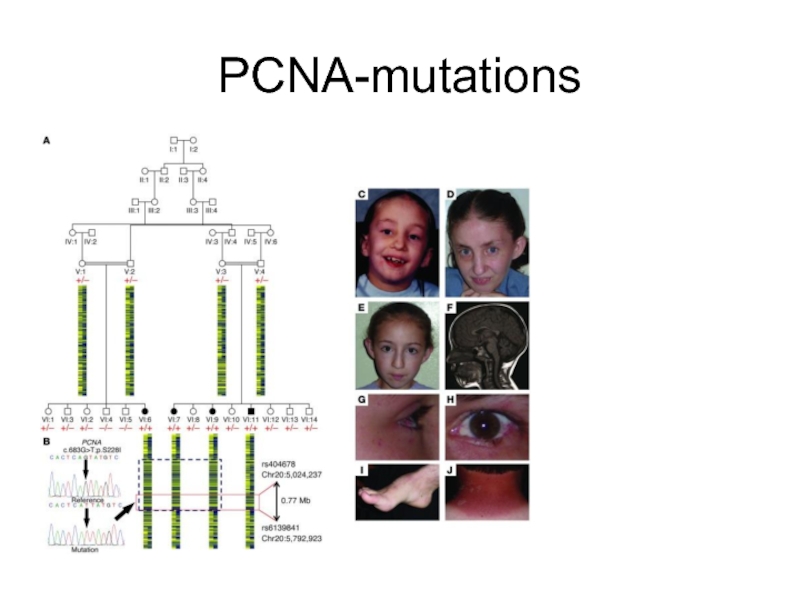
- 55. PCNA-mutations
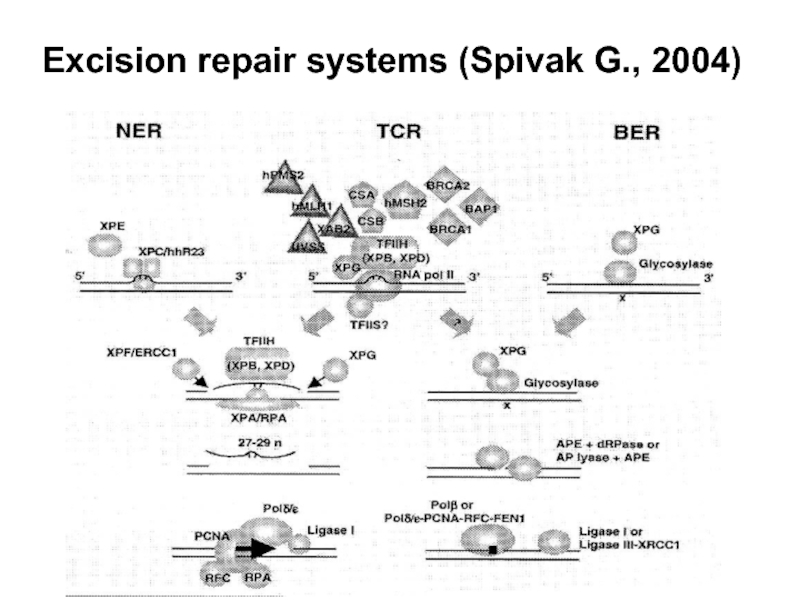
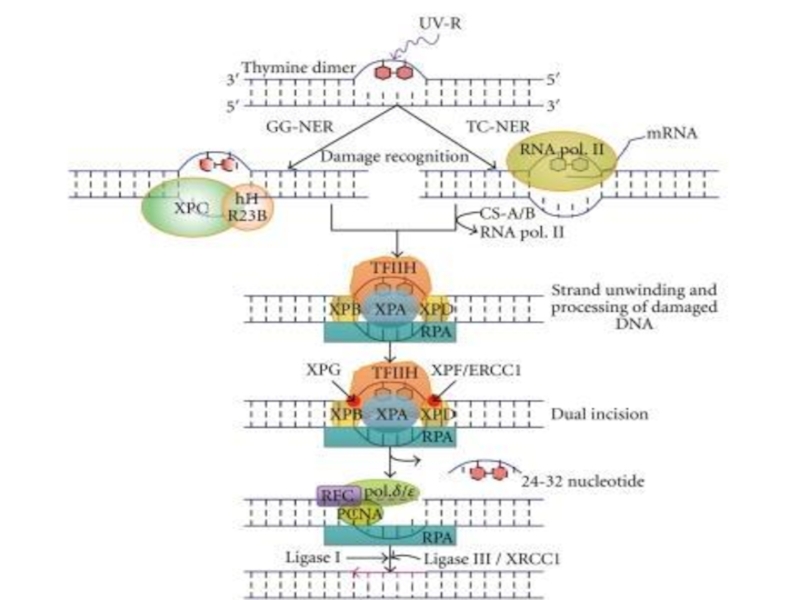
- 56. Excision repair systems (Spivak G., 2004)
- 58. Xeroderma pigmentosum (XP) XPA, XPB, XPD, XPC,
- 59. Lai et al., 2013. The influence of
- 60. Autosomal recessive CSA (WD-repeat protein, β-subunit
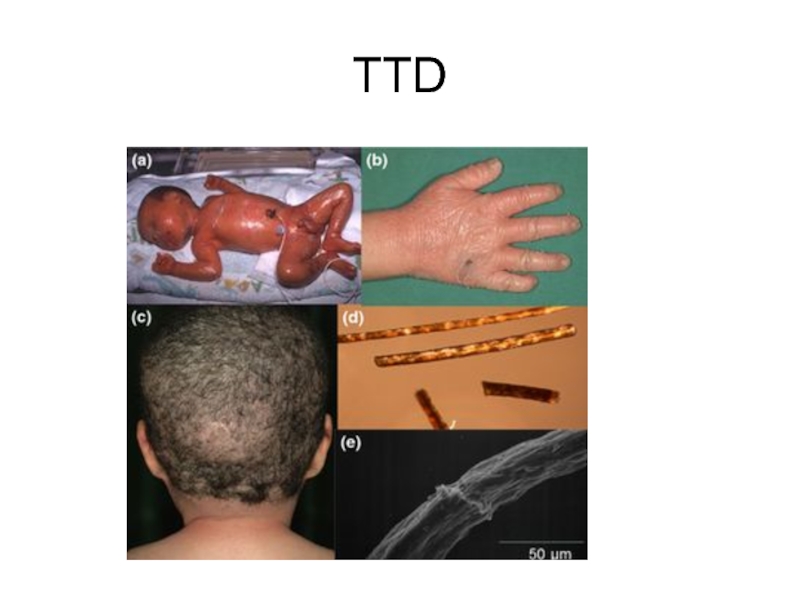
- 61. TTD
- 62. Анемия Фанкони FAA, FAB, FAC, FAD, FAE,
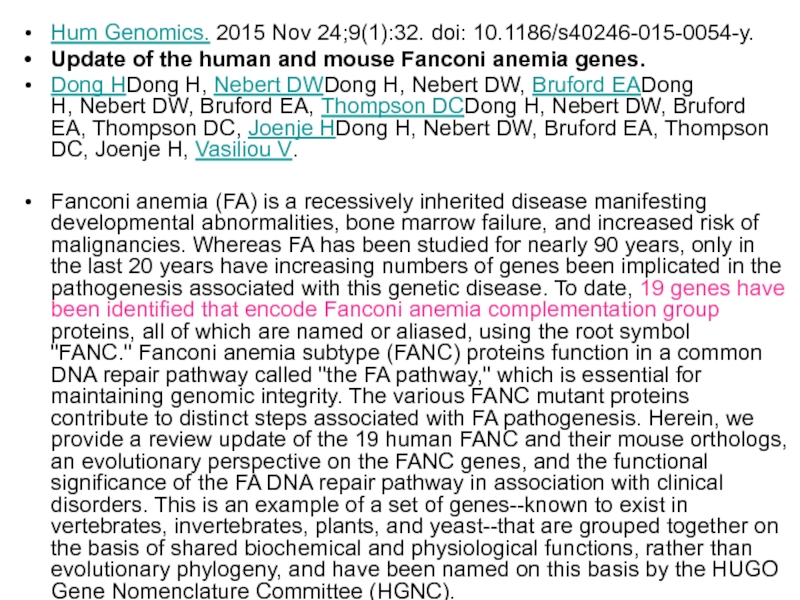
- 63. Hum Genomics. 2015 Nov 24;9(1):32. doi: 10.1186/s40246-015-0054-y. Update
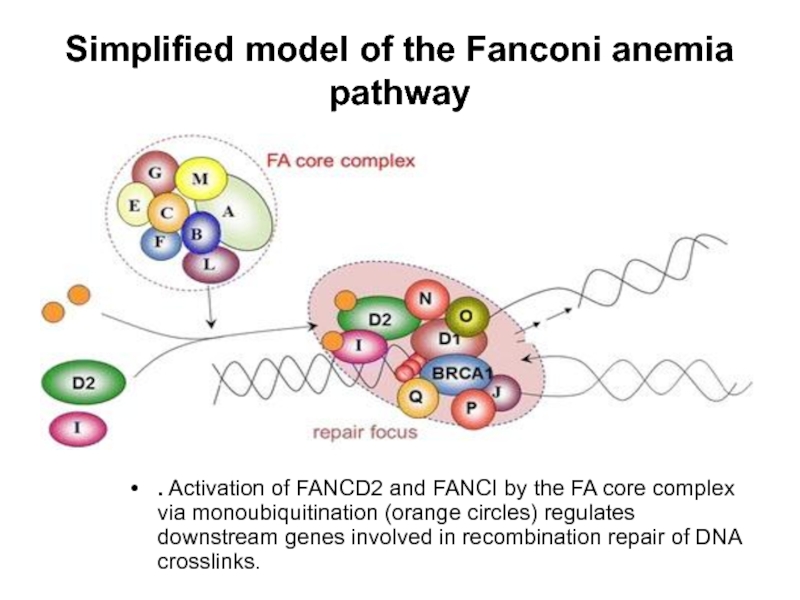
- 64. Simplified model of the Fanconi anemia pathway
- 67. Age of onset of SCC in FA
- 68. Telomeres Telomeres, being the final fragments of
- 69. Age-dependent telomere length
- 70. Telomere length
- 71. Factor analysis of mean telomere length, genetic
- 72. Запретить
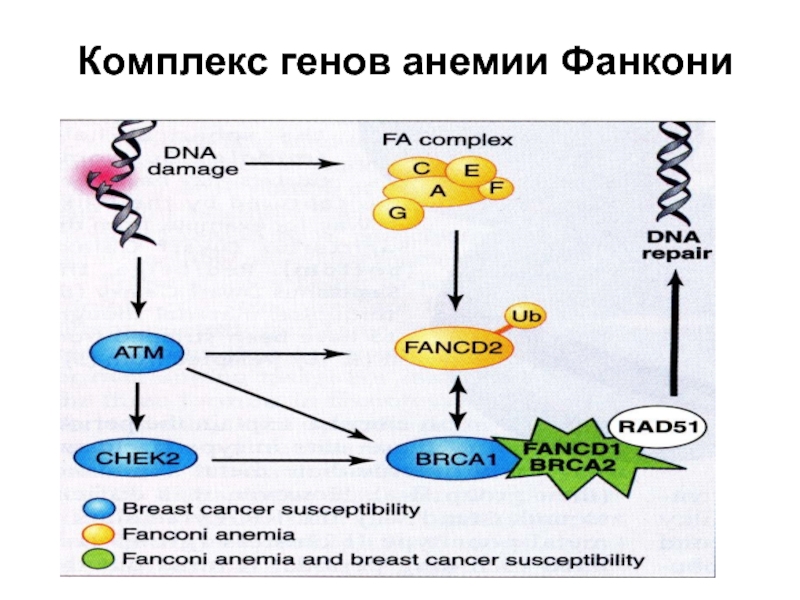
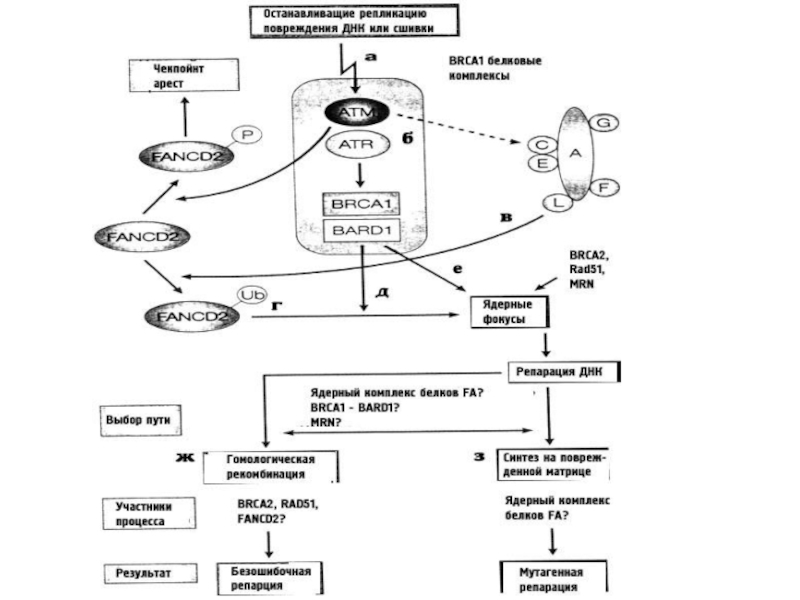
- 74. Комплекс генов анемии Фанкони
- 75. Simplified model of the Fanconi anemia pathway
- 76. Mechanism of ICL repair in the FA
- 77. FA/BRCA pathway and crosstalk between FA and
- 78. Overview of FA pathway genes identified in
- 79. Domain architecture and structure of FANCD2 and
- 80. Comparison of the SCF multi-subunit ubiquitin ligase
- 81. Models for FANCD2 and FANCI monoubiquitination The
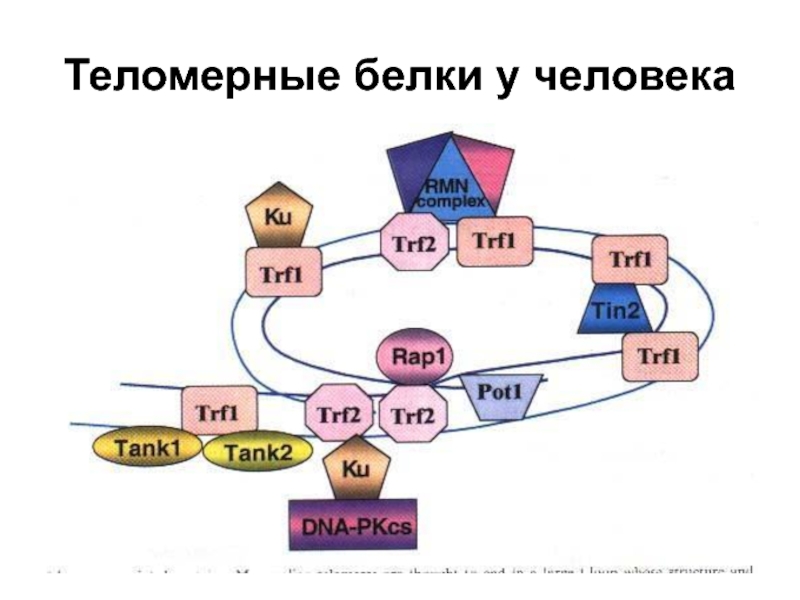
- 82. Теломерные белки у человека
- 83. Data acquired in the course of study
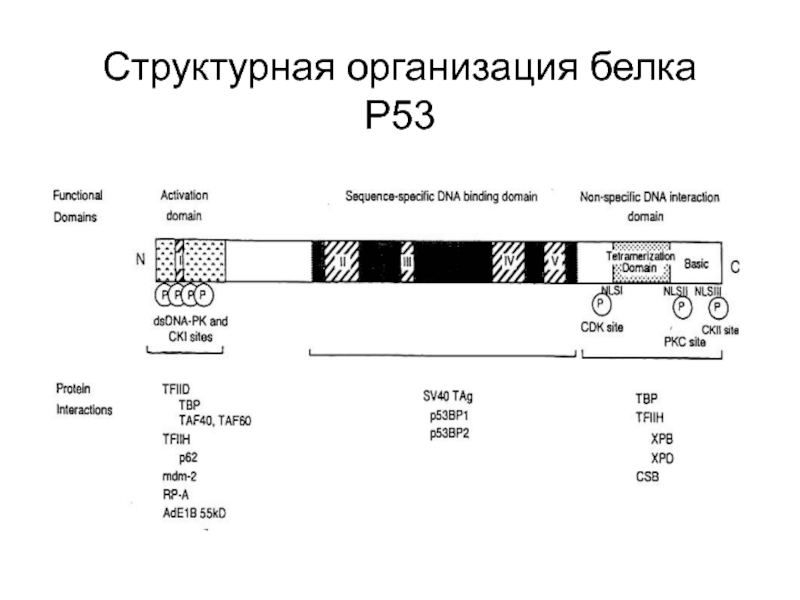
- 84. Структурная организация белка Р53
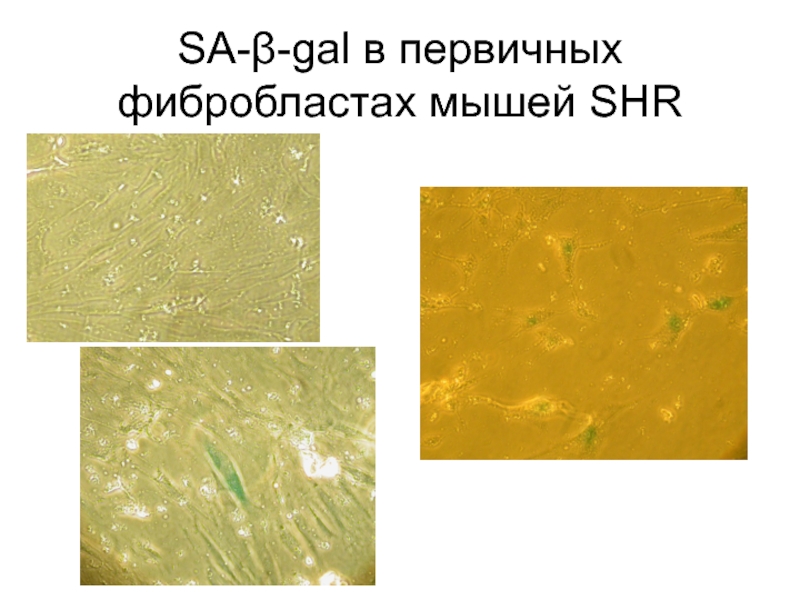
- 85. SA-β-gal в первичных фибробластах мышей SHR
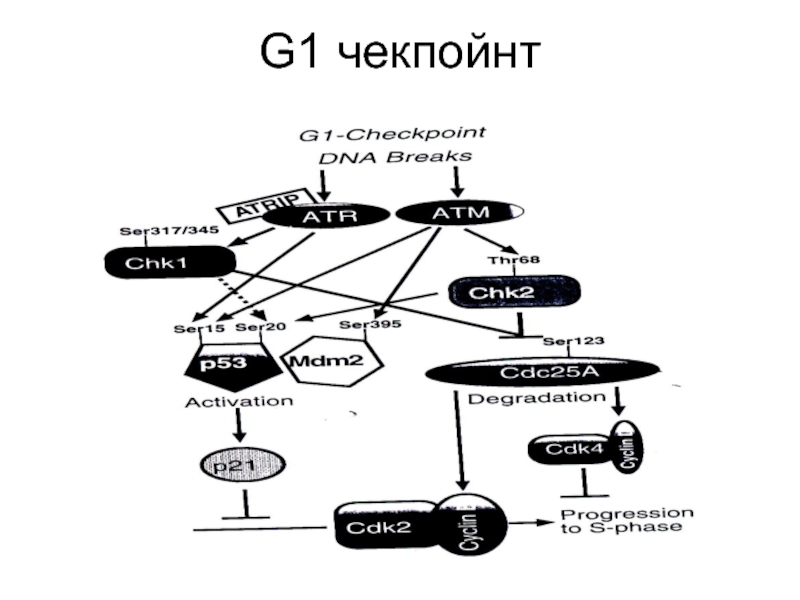
- 86. G1 чекпойнт
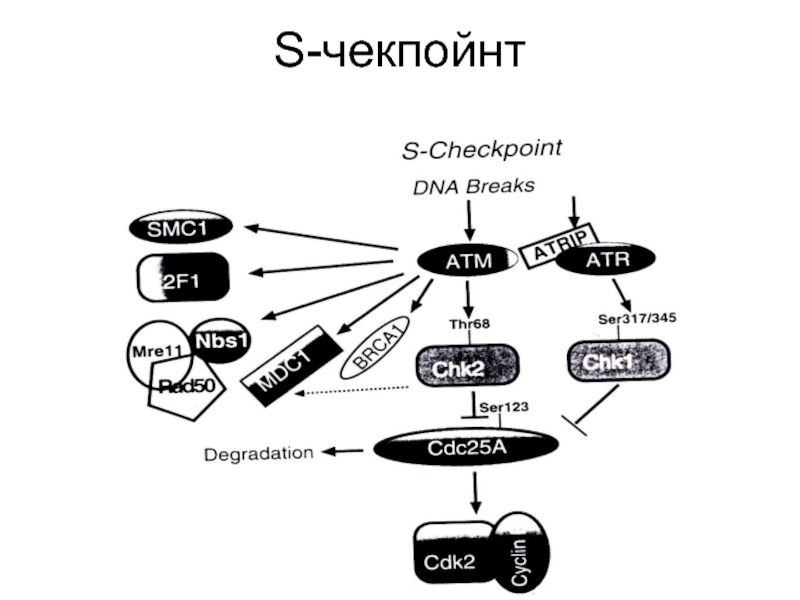
- 87. S-чекпойнт
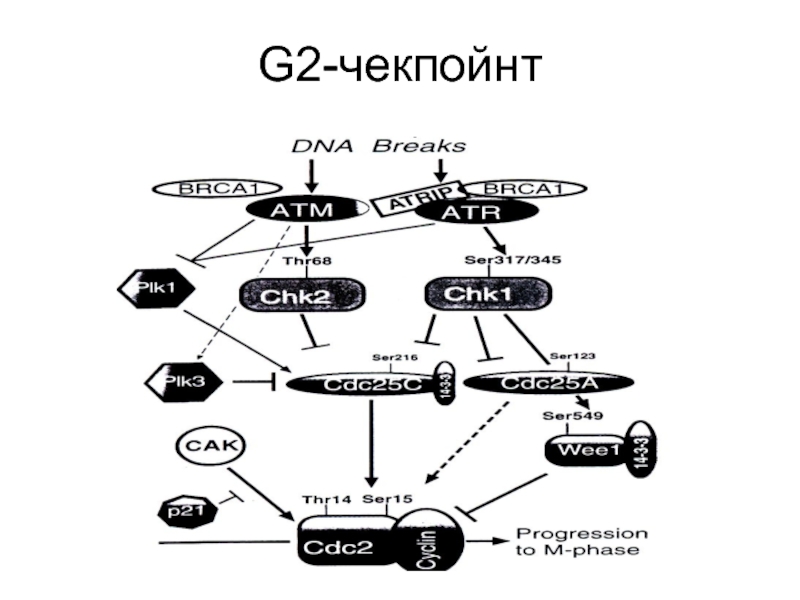
- 88. G2-чекпойнт
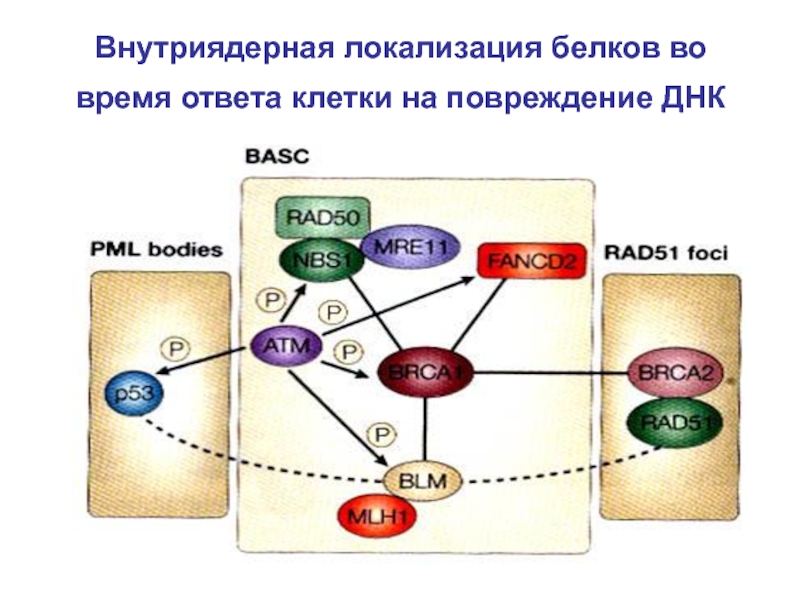
- 89. Внутриядерная локализация белков во время ответа клетки на повреждение ДНК
- 90. Механизмы G2-ареста АТМ активирует СНК2 через фосфорелирование
- 91. Механизмы G2-ареста(2) После облучения резко падает уровень
- 92. Механизмы G2-ареста(3) PLK1 и PLK3 (Polo-like kinase).
- 93. Механизмы G2-ареста(4) Остановить вход в митоз при
- 94. Механизмы G2-ареста(5) Роль Р53 в поддержании G2-ареста
- 95. Механизмы G2-ареста(6) G2 чекпойнт-ответ разделяется на два
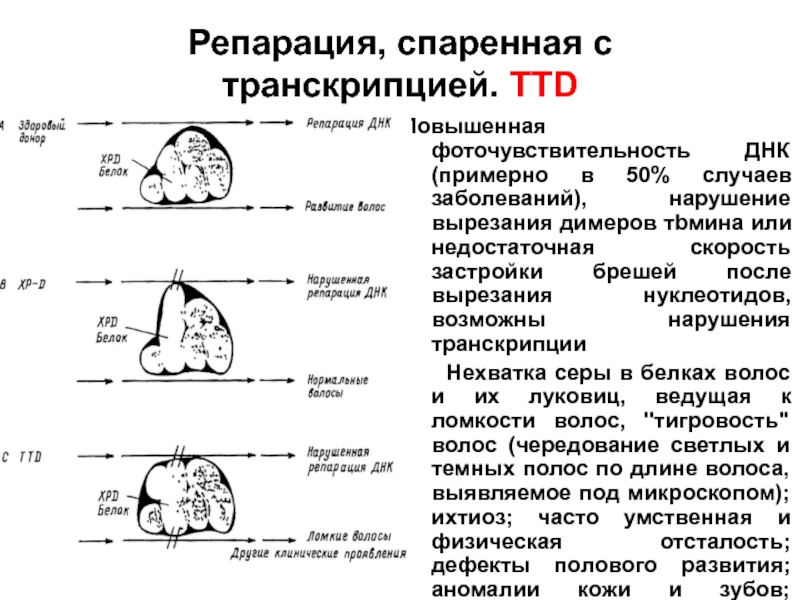
- 96. Репарация, спаренная с транскрипцией. TTD Повышенная фоточувствительность
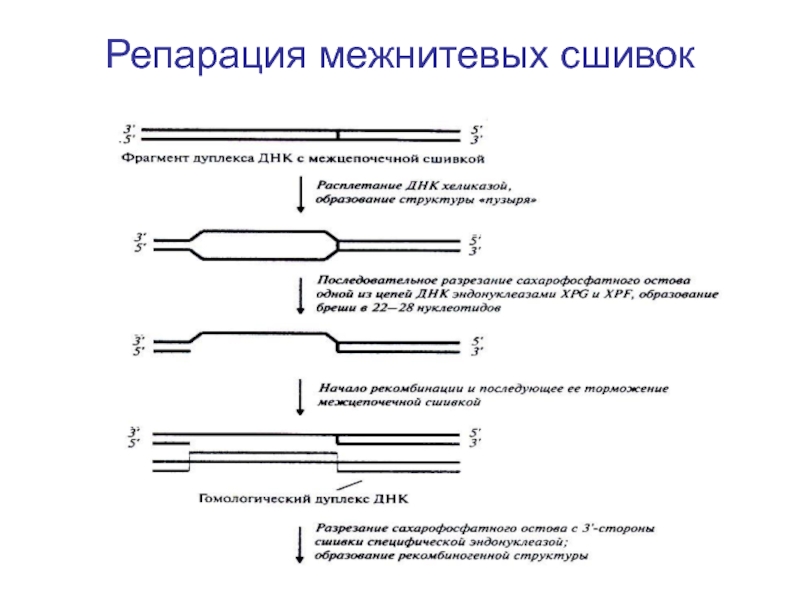
- 97. Репарация межнитевых сшивок Схема процеса репарации сшивок
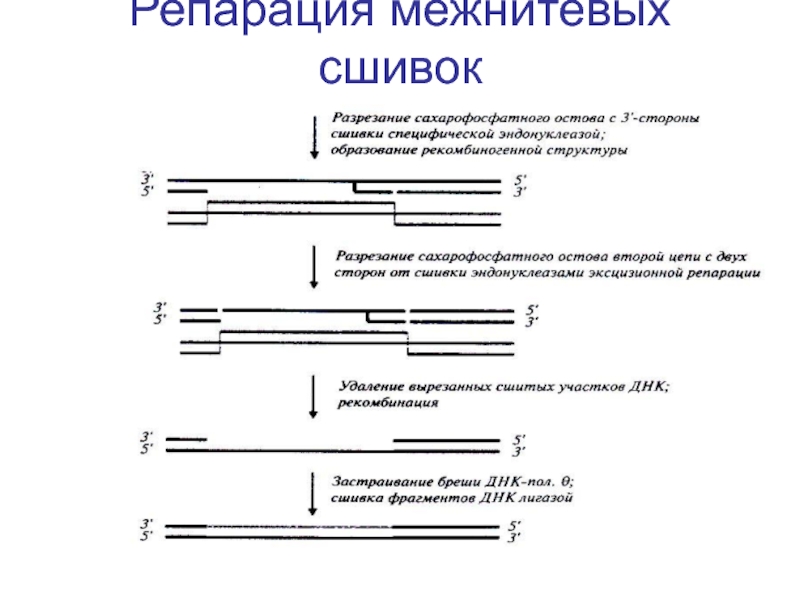
- 98. Репарация межнитевых сшивок
- 99. Репарация межнитевых сшивок
Слайд 1Accelerated aging diseases and their genetic causes Complex pattern of aging
Слайд 2Primary non-transformed cell cultures tend to change with every passage. Population
Слайд 5The most frequently used marker of aging is lysosomal β-galactosidase (SA-β-gal)
Слайд 6Primary fibroblasts of skin from donors of varios ages, and patients
Fibroblasts taken from old donors, and from the progeria patients, are currently regarded as containing more ageing markers, than those taken from young healthy donors (Scafidi, Misteli, 2006, Sedelnikova et al., 2008).
Fibroblasts of skin of other mammals (mice) may also be applied for the detection of ageing markers (taking into account the Hyflick limit)
Слайд 7Hutchinson-Gilford syndrome
Autosomal dominant, emerging “de novo”, getting, probably, from the father
15 (21)
LMNA 1q21.2
FACE-1/ZMPSTE24 1q34
Skin atrophy.
Bird’s face
Loss of subcutaneous fat
Hair loss.
Arteriosclerosis
Increased metabolic rate
Hypogonadism
Grow hormone insensitivity?
Slow growth.
Reduced replicative life span of cultured cells
Shot telomeres
DNA repair defects?
Silent mutation in (1-4)-β-Galactosyl-transferase gene (594C>T)
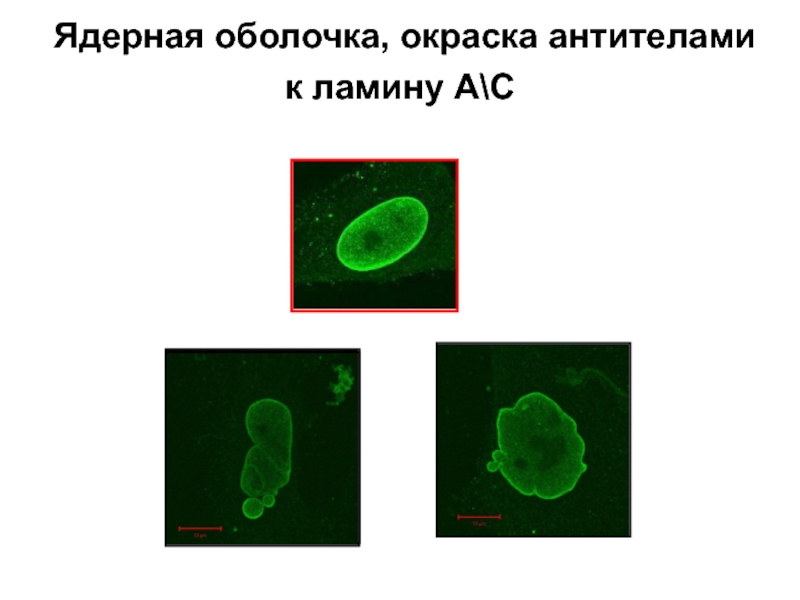
Слайд 9Nuclear lamina
Structural transformations of the nuclear lamina, occurring as a result
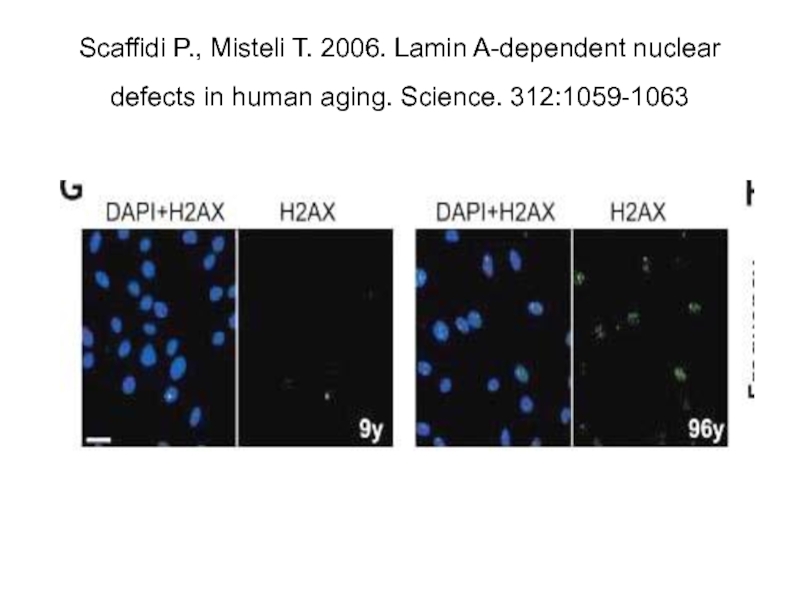
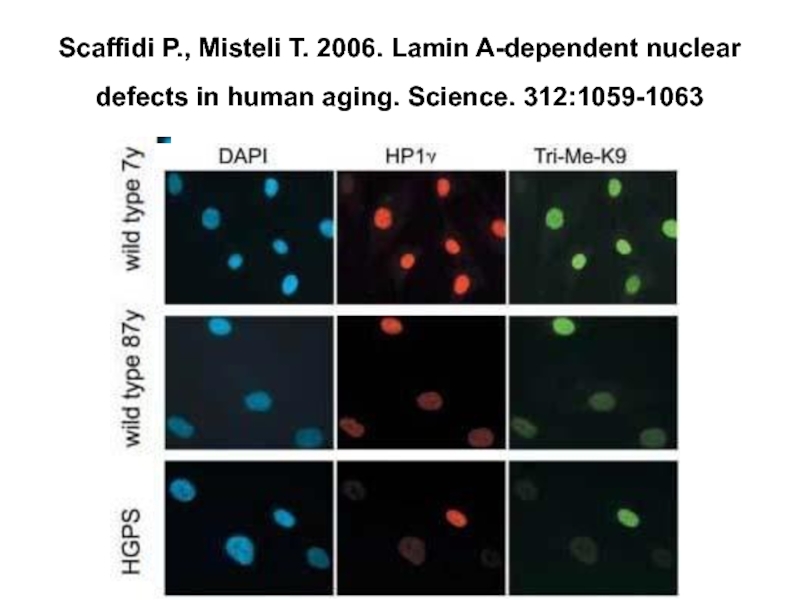
Слайд 10 Scaffidi P., Misteli T. 2006. Lamin A-Dependent Nuclear Defects in Human
Farnezyl-protein-transpherase
Ras-converting enzyme or Zmpste24
S-adenosyl- metionin;
isiprenyl-carboxymethyl-transpherase
Zmpste24
lamine А
«progerin»
Rusinol,
Sinensky, 2006
Слайд 12 Chen et al., 2003. LMNA mutations in atypical Werner’s syndrome. Lancet
Ramirez et al., 2006
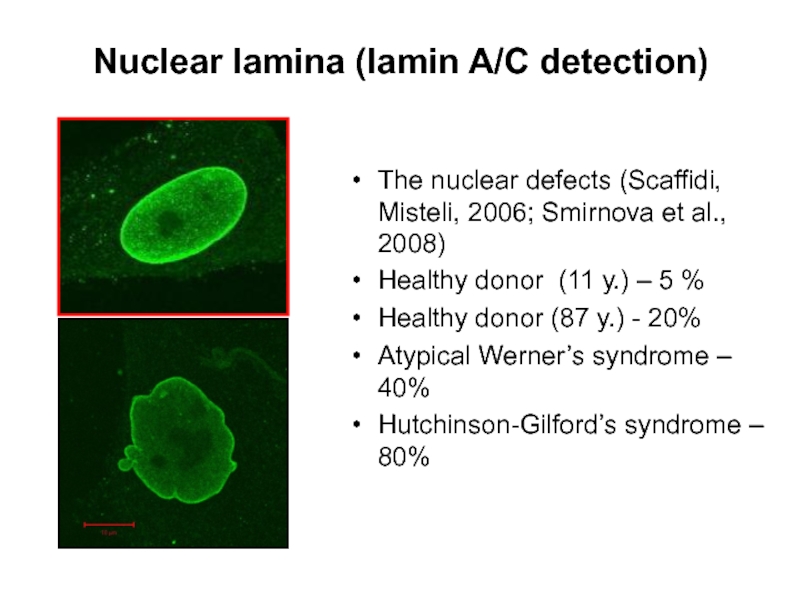
Слайд 14Nuclear lamina (lamin A/C detection)
The nuclear defects (Scaffidi, Misteli, 2006; Smirnova
Healthy donor (11 у.) – 5 %
Healthy donor (87 y.) - 20%
Atypical Werner’s syndrome – 40%
Hutchinson-Gilford’s syndrome – 80%
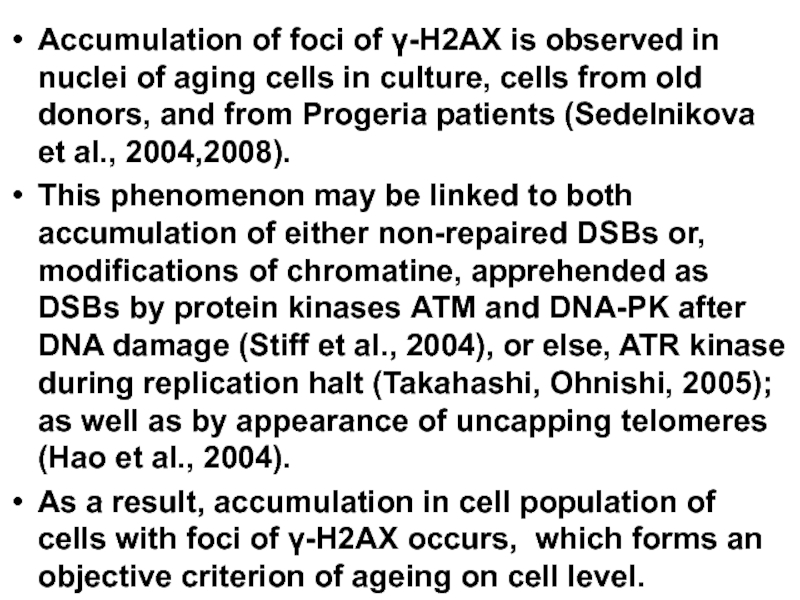
Слайд 15Accumulation of foci of γ-Н2АХ is observed in nuclei of aging
This phenomenon may be linked to both accumulation of either non-repaired DSBs or, modifications of chromatine, apprehended as DSBs by protein kinases АТМ and DNA-PK after DNA damage (Stiff et al., 2004), or else, ATR kinase during replication halt (Takahashi, Ohnishi, 2005); as well as by appearance of uncapping telomeres (Hao et al., 2004).
As a result, accumulation in cell population of cells with foci of γ-Н2АХ occurs, which forms an objective criterion of ageing on cell level.
Слайд 16Scaffidi P., Misteli T. 2006. Lamin A-dependent nuclear defects in human
Слайд 17Cells change their epigenetic status with age. Heritable changes in gene
Слайд 18Scaffidi P., Misteli T. 2006. Lamin A-dependent nuclear defects in human
Слайд 19Werner syndrome
Autosomal recessive
53 (60)
Loss of WRN,
Skin atrophy.
Hair graying/loss.
Arteriosclerosis
Osteoporosis
Muscle atrophy
Cataracts
Hyperlipidemia
Mild diabetes melitus, type 2
Hypogonadism
Cancer (sarcomas)
Slow growth.
Reduced replicative life span of cultured cells
Chromosome rearrangements
Sensitivity to 4-NQO and camptothecin (topoisomerase I poison)
Increased mutation rate, particulary DNA deletions
Rapid telomere shortening during cellular life span.
DNA repair defects
DNA replication defects (?)
Слайд 22Autosomal recessive
20 (40-50)
BLM-helicase (RecQ-homologue)
Increased metabolic rate
Slow growth.
Diabetes melitus
Hypogonadism
Neurodegeneration
Immunodeficiency
Cancer
Telangiectasias on
Low DNA synthesis (2 times) with the normal rate of DNA-polymerases α, β, γ
Chromosomes rearrangements
Icreased level of recombination
Bloom syndrome
Слайд 26In the study of cells from a patient with Hutchinson-Gilford syndrome,
Слайд 27 The stable chromosome (1, 4, 8 or 12) aberration (SCA)
Age dynamics
The patients with Werner’s syndrome demonstrate increased SCA level, corresponding to premature aging of organisms.
The lymphocytes from patient with Hutchinson-Gilford syndrome have no time for SCA formation
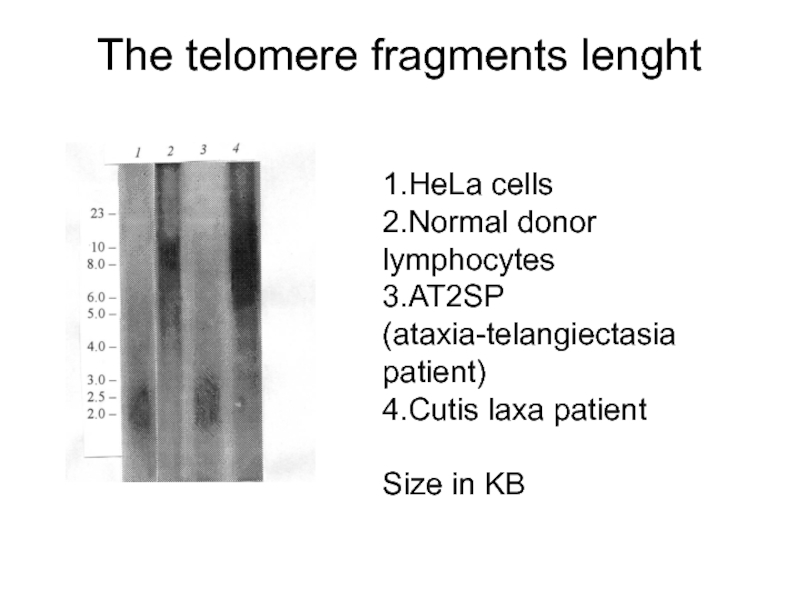
Слайд 29The telomere fragments lenght
1.HeLa cells
2.Normal donor lymphocytes
3.AT2SP (ataxia-telangiectasia patient)
4.Cutis laxa
Size in KB
Слайд 32Autosomal recessive
20 (40-50)
ATM, a protein kinase of the PI-3 kinase family
Neurodegeneration
Immunodeficiency
Cancer
Occulocutaneous telangiectasias
Progeroid skin and hear changes
Hypogonadism
Defectif cell cycle chekpoint arrest
Reduced replicative life span
Chromosomal rearrangements
Sensitivity to ionizing radiation
Inoppropriate apoptosis
Shot telomeres
Delayed/absence P53 induction and accumulation after DNA damage
Defects in repair of DNA double-strand breaks
Defects in V(D)J recombination
Ataxia-telangiectasia (Luis-Bar syndrome)
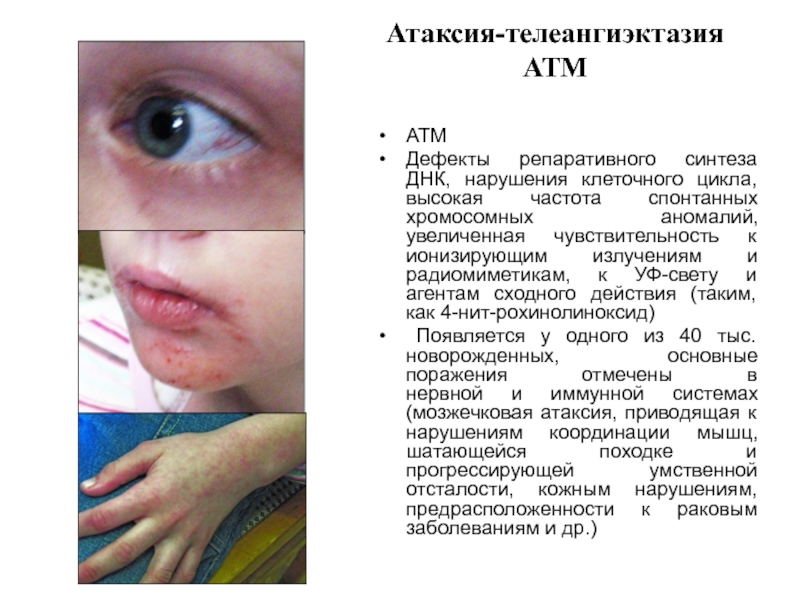
Слайд 33Атаксия-телеангиэктазия АТМ
АТМ
Дефекты репаративного синтеза ДНК, нарушения клеточного цикла, высокая частота спонтанных
Появляется у одного из 40 тыс. новорожденных, основные поражения отмечены в нервной и иммунной системах (мозжечковая атаксия, приводящая к нарушениям координации мышц, шатающейся походке и прогрессирующей умственной отсталости, кожным нарушениям, предрасположенности к раковым заболеваниям и др.)
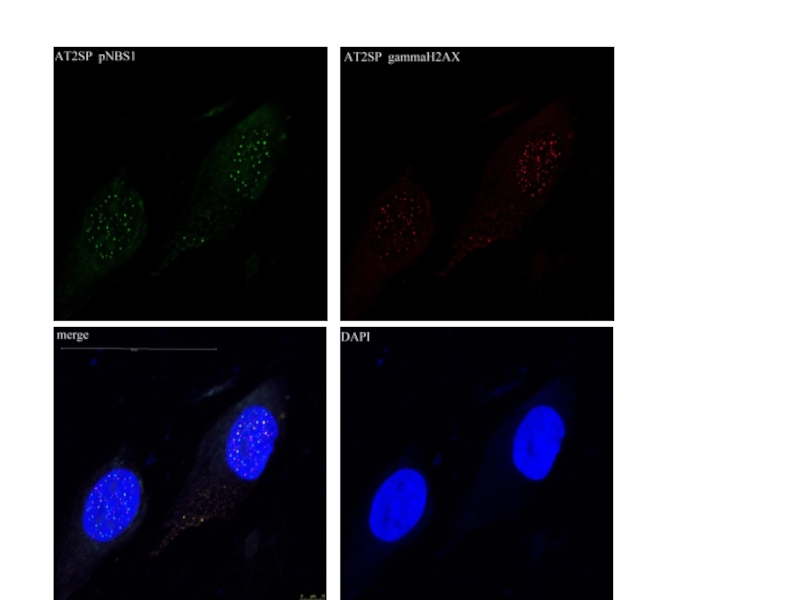
Слайд 37Detection of γ-Н2АХ in nuclei of human fibroblasts VH-10 (11 y),
1.08±1.07
4.06±1.28*
12.32±2.09**
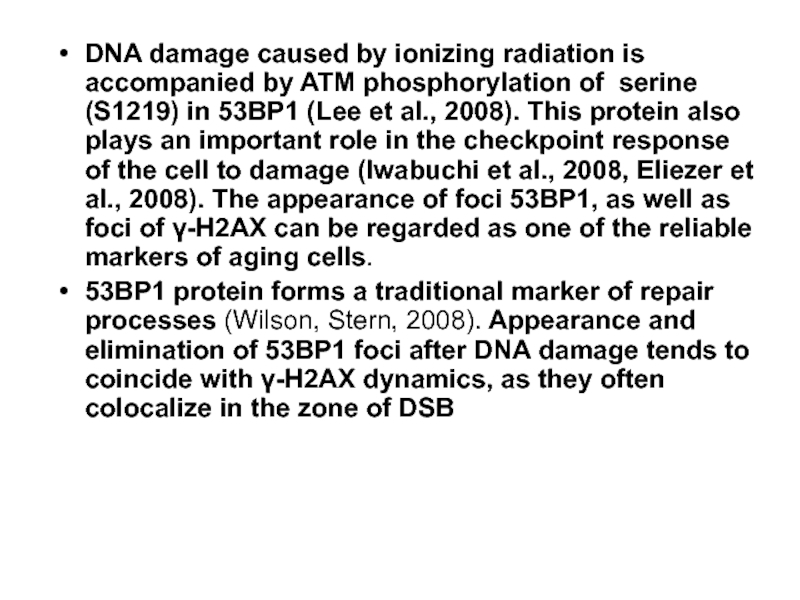
Слайд 40DNA damage caused by ionizing radiation is accompanied by ATM phosphorylation
53ВР1 protein forms a traditional marker of repair processes (Wilson, Stern, 2008). Appearance and elimination of 53ВР1 foci after DNA damage tends to coincide with γ-Н2АХ dynamics, as they often colocalize in the zone of DSB
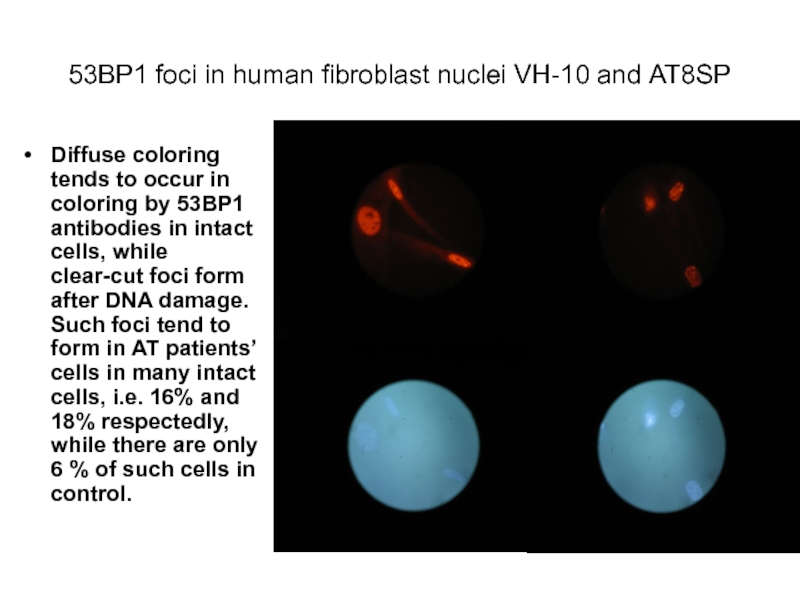
Слайд 4153ВР1 foci in human fibroblast nuclei VH-10 and АТ8SP
Diffuse coloring tends
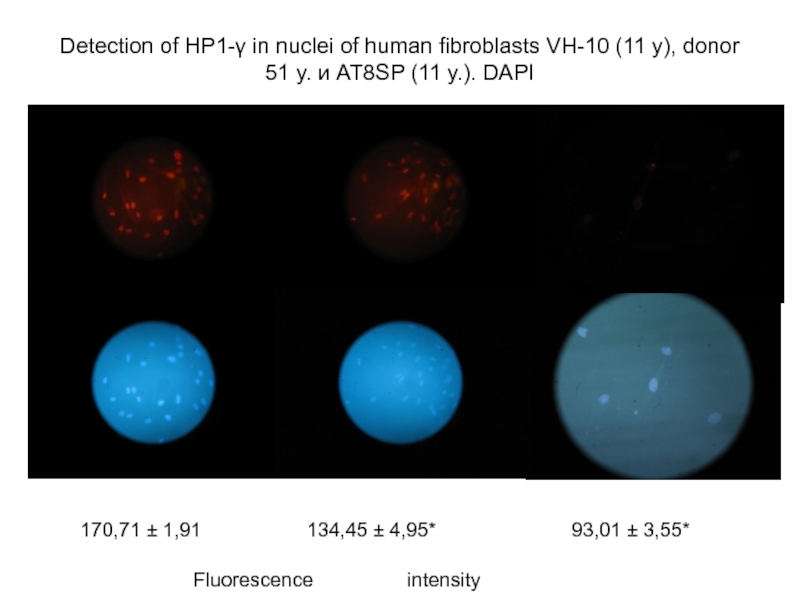
Слайд 42Detection of НР1-γ in nuclei of human fibroblasts VH-10 (11 y),
170,71 ± 1,91
134,45 ± 4,95*
93,01 ± 3,55*
Fluorescence intensity
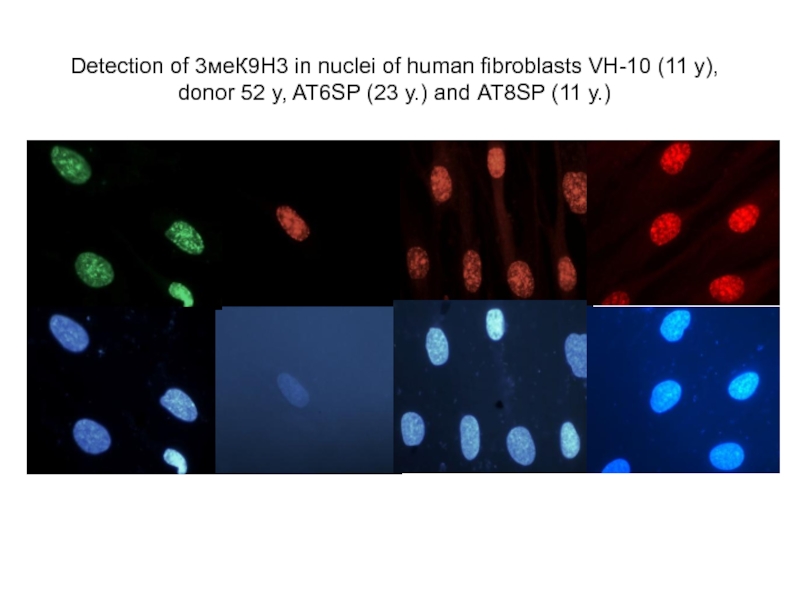
Слайд 44Detection of 3меК9Н3 in nuclei of human fibroblasts VH-10 (11 y),
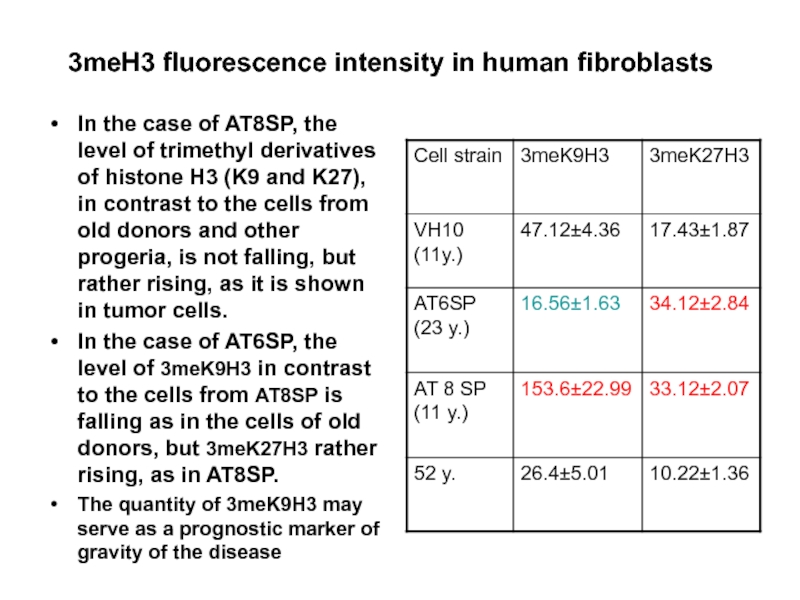
Слайд 453meH3 fluorescence intensity in human fibroblasts
In the case of AT8SP, the
In the case of AT6SP, the level of 3meK9H3 in contrast to the cells from AT8SP is falling as in the cells of old donors, but 3meK27H3 rather rising, as in AT8SP.
The quantity of 3meK9H3 may serve as a prognostic marker of gravity of the disease
Слайд 46Seckel syndrome (atr)
O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship
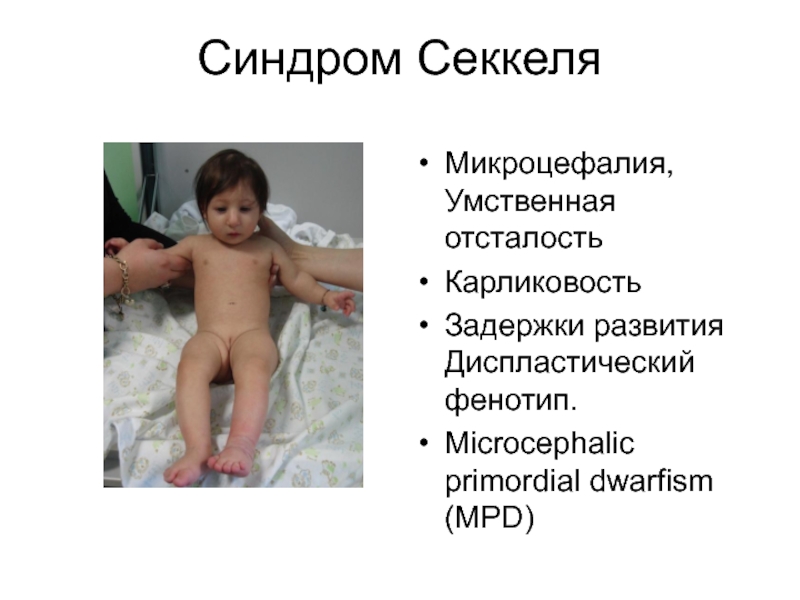
Слайд 47Синдром Секкеля
Микроцефалия, Умственная отсталость
Карликовость
Задержки развития Диспластический фенотип.
Microcephalic primordial dwarfism (MPD)
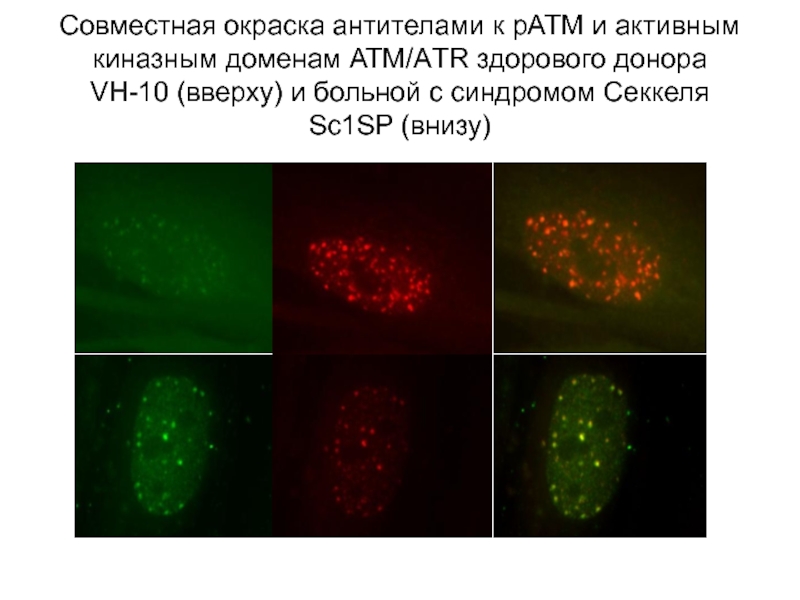
Слайд 48Совместная окраска антителами к рАТМ и активным киназным доменам АТМ/АTR здорового
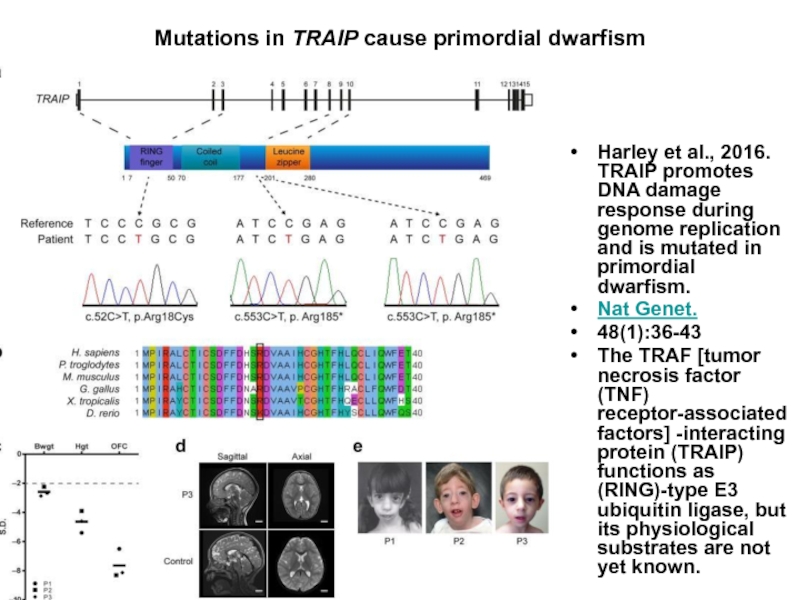
Слайд 53Mutations in TRAIP cause primordial dwarfism
Harley et al., 2016. TRAIP promotes DNA damage
Nat Genet.
48(1):36-43
The TRAF [tumor necrosis factor (TNF) receptor-associated factors] -interacting protein (TRAIP) functions as (RING)-type E3 ubiquitin ligase, but its physiological substrates are not yet known.
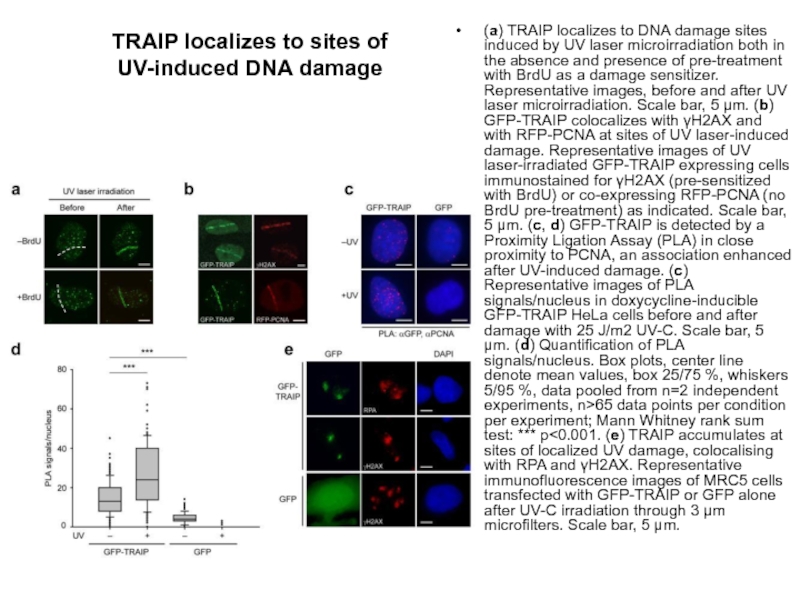
Слайд 54TRAIP localizes to sites of UV-induced DNA damage
(a) TRAIP localizes to
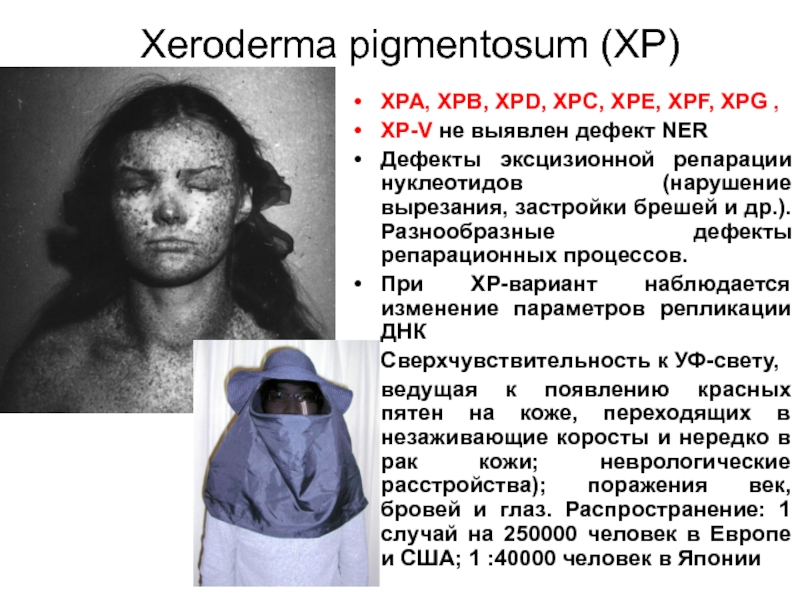
Слайд 58Xeroderma pigmentosum (XP)
XPA, XPB, XPD, XPC, XPE, XPF, XPG ,
XP-V не
Дефекты эксцизионной репарации нуклеотидов (нарушение вырезания, застройки брешей и др.). Разнообразные дефекты репарационных процессов.
При XP-вариант наблюдается изменение параметров репликации ДНК
Сверхчувствительность к УФ-свету,
ведущая к появлению красных пятен на коже, переходящих в незаживающие коросты и нередко в рак кожи; неврологические расстройства); поражения век, бровей и глаз. Распространение: 1 случай на 250000 человек в Европе и США; 1 :40000 человек в Японии
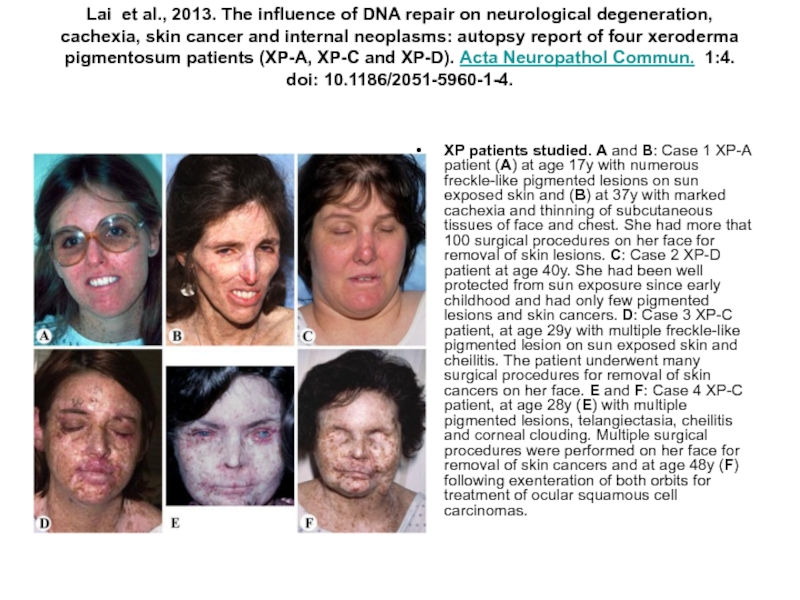
Слайд 59Lai et al., 2013. The influence of DNA repair on neurological degeneration, cachexia,
XP patients studied. A and B: Case 1 XP-A patient (A) at age 17y with numerous freckle-like pigmented lesions on sun exposed skin and (B) at 37y with marked cachexia and thinning of subcutaneous tissues of face and chest. She had more that 100 surgical procedures on her face for removal of skin lesions. C: Case 2 XP-D patient at age 40y. She had been well protected from sun exposure since early childhood and had only few pigmented lesions and skin cancers. D: Case 3 XP-C patient, at age 29y with multiple freckle-like pigmented lesion on sun exposed skin and cheilitis. The patient underwent many surgical procedures for removal of skin cancers on her face. E and F: Case 4 XP-C patient, at age 28y (E) with multiple pigmented lesions, telangiectasia, cheilitis and corneal clouding. Multiple surgical procedures were performed on her face for removal of skin cancers and at age 48y (F) following exenteration of both orbits for treatment of ocular squamous cell carcinomas.
Слайд 60Autosomal recessive
CSA (WD-repeat protein, β-subunit GTP protein homologue)
CSB (ATPase of Swi2
XPB and XPD (helicases in TFII-H),
XPG (yeast Rad2 homologue, endonuclease)
20 (40?)
Bird’s face
Loss of subcutaneous fat
Skin photosensitivity
Neurodegeneration
Hypogonadism
UV-sensitivity
Impaired transcription-coupled nucleotide excision repair
Decreased recovery of transcription after irradiation
General RNA-polymerase II transcription defects
Cockayne syndrome (CS)
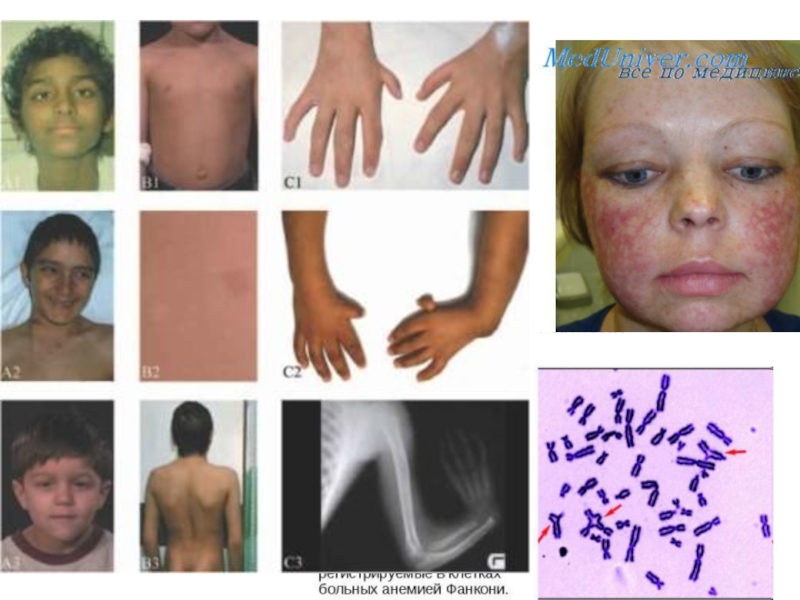
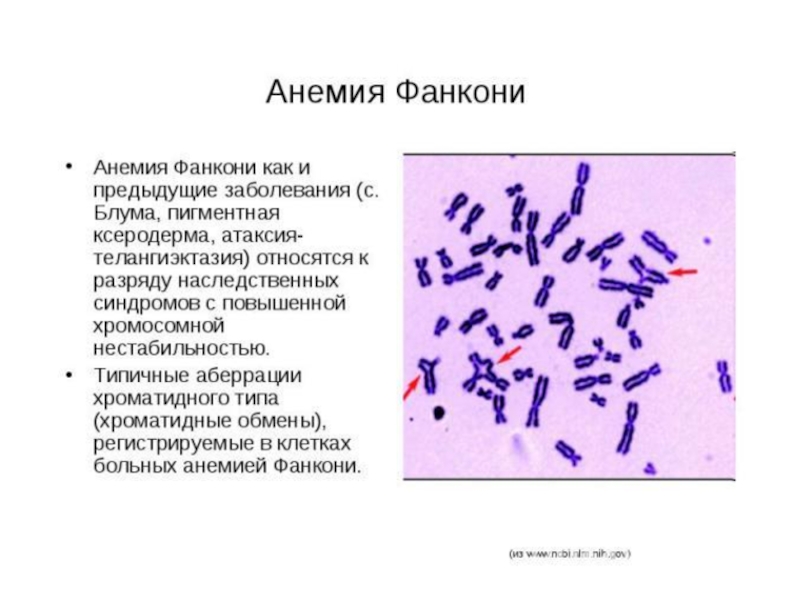
Слайд 62Анемия Фанкони
FAA, FAB, FAC, FAD, FAE, FAF, FAG -19 групп комплементации
Дефекты
пониженная способность к апоптозу после ионизирующего облучения; двукратное удлинение G2-фазы клеточного цикла
Сверхчувствительность к химическим мутагенам и канцерогенам, уменьшение количества всех клеточных элементов крови, различные аномалии врожденных способностей, деформация пальцев и другие виды скелетных нарушений, урогенитальные нарушения, микроцефалия, микрофтальмия, дефекты уха и потеря слуха, сердечные и гастроинтестинальные нарушения
Слайд 63Hum Genomics. 2015 Nov 24;9(1):32. doi: 10.1186/s40246-015-0054-y.
Update of the human and mouse Fanconi
Dong HDong H, Nebert DWDong H, Nebert DW, Bruford EADong H, Nebert DW, Bruford EA, Thompson DCDong H, Nebert DW, Bruford EA, Thompson DC, Joenje HDong H, Nebert DW, Bruford EA, Thompson DC, Joenje H, Vasiliou V.
Fanconi anemia (FA) is a recessively inherited disease manifesting developmental abnormalities, bone marrow failure, and increased risk of malignancies. Whereas FA has been studied for nearly 90 years, only in the last 20 years have increasing numbers of genes been implicated in the pathogenesis associated with this genetic disease. To date, 19 genes have been identified that encode Fanconi anemia complementation group proteins, all of which are named or aliased, using the root symbol "FANC." Fanconi anemia subtype (FANC) proteins function in a common DNA repair pathway called "the FA pathway," which is essential for maintaining genomic integrity. The various FANC mutant proteins contribute to distinct steps associated with FA pathogenesis. Herein, we provide a review update of the 19 human FANC and their mouse orthologs, an evolutionary perspective on the FANC genes, and the functional significance of the FA DNA repair pathway in association with clinical disorders. This is an example of a set of genes--known to exist in vertebrates, invertebrates, plants, and yeast--that are grouped together on the basis of shared biochemical and physiological functions, rather than evolutionary phylogeny, and have been named on this basis by the HUGO Gene Nomenclature Committee (HGNC).
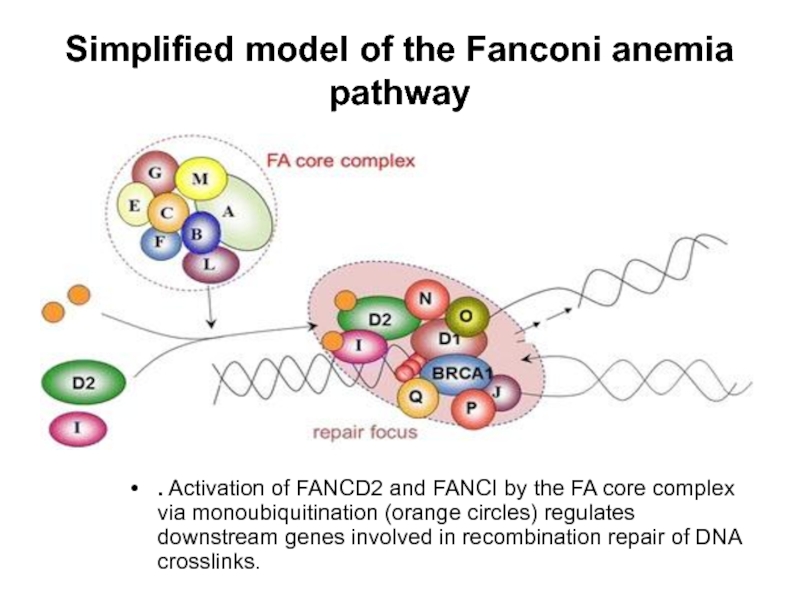
Слайд 64Simplified model of the Fanconi anemia pathway
. Activation of FANCD2 and FANCI
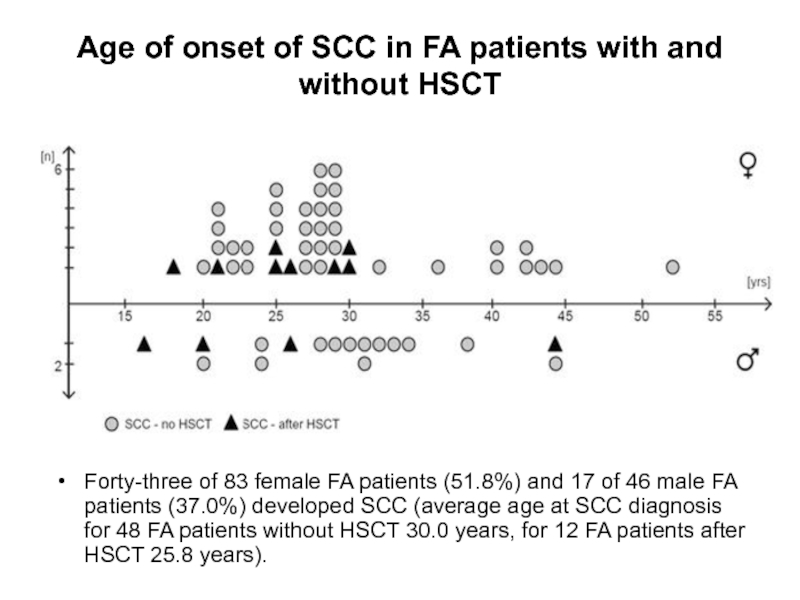
Слайд 67Age of onset of SCC in FA patients with and without
Forty-three of 83 female FA patients (51.8%) and 17 of 46 male FA patients (37.0%) developed SCC (average age at SCC diagnosis for 48 FA patients without HSCT 30.0 years, for 12 FA patients after HSCT 25.8 years).
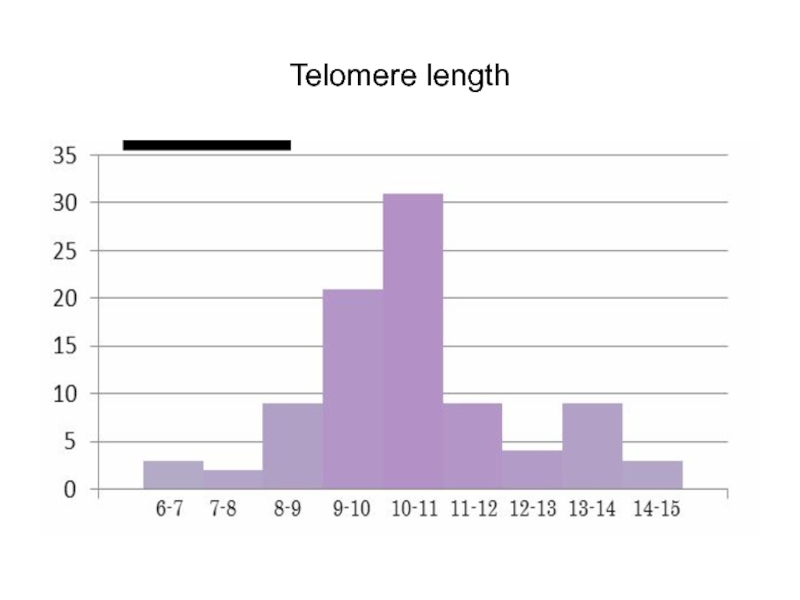
Слайд 68Telomeres
Telomeres, being the final fragments of eukaryotic chromosomes, form one of
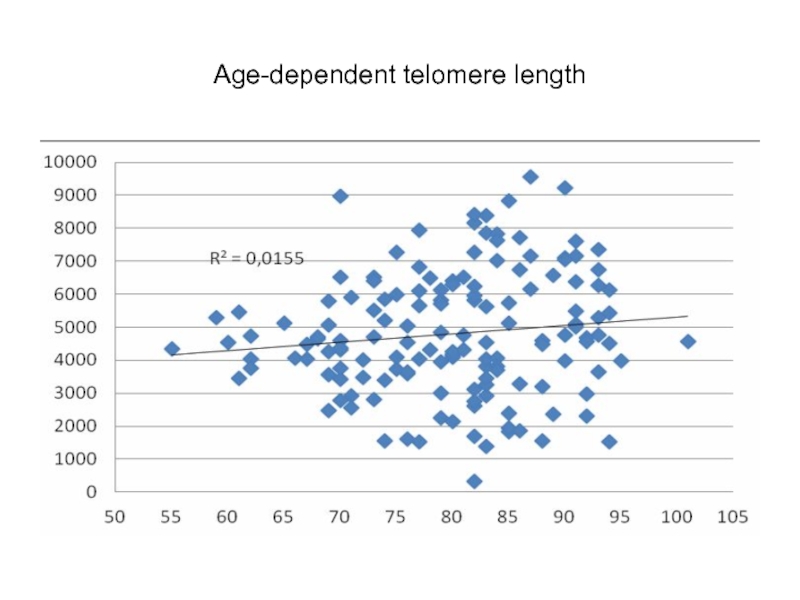
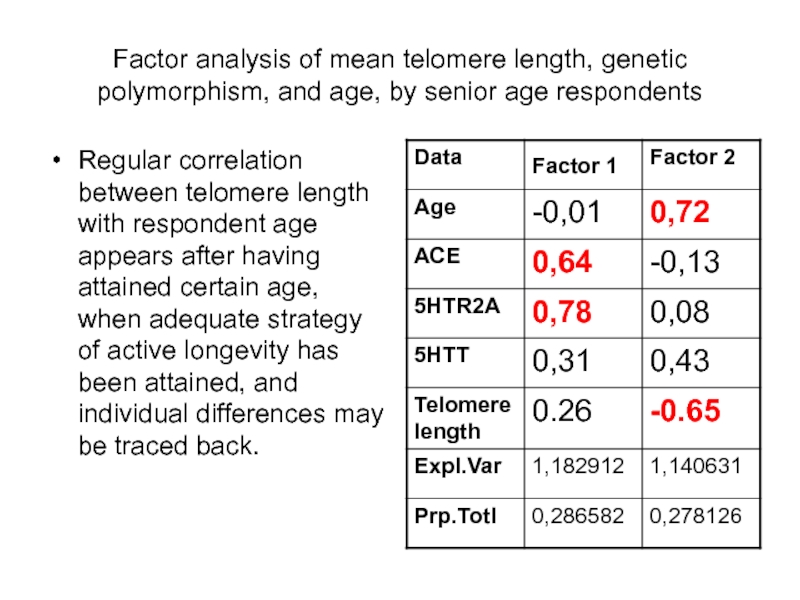
Слайд 71Factor analysis of mean telomere length, genetic polymorphism, and age, by
Regular correlation between telomere length with respondent age appears after having attained certain age, when adequate strategy of active longevity has been attained, and individual differences may be traced back.
Слайд 75Simplified model of the Fanconi anemia pathway
. Activation of FANCD2 and FANCI
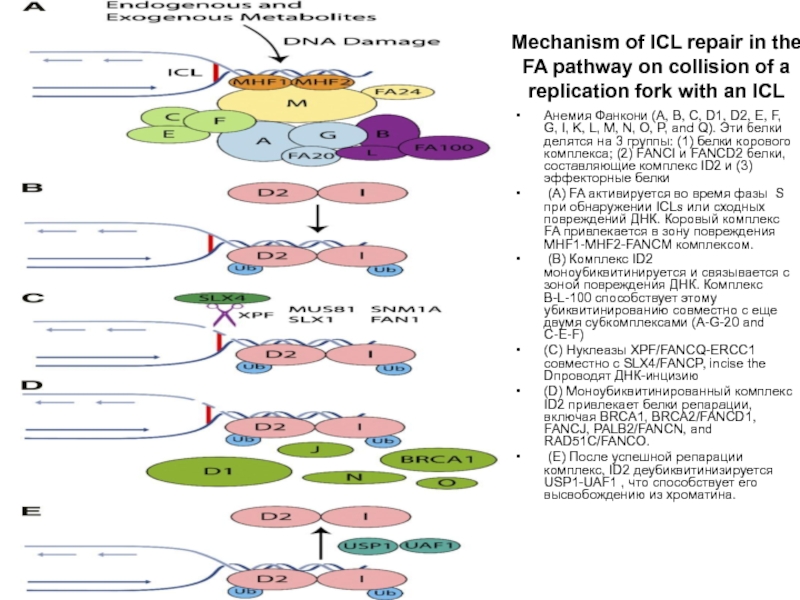
Слайд 76Mechanism of ICL repair in the FA pathway on collision of
Анемия Фанкони (A, B, C, D1, D2, E, F, G, I, K, L, M, N, O, P, and Q). Эти белки делятся на 3 группы: (1) белки корового комплекса; (2) FANCI и FANCD2 белки, составляющие комплекс ID2 и (3) эффекторные белки
(A) FA активируется во время фазы S при обнаружении ICLs или сходных повреждений ДНК. Коровый комплекс FA привлекается в зону повреждения MHF1-MHF2-FANCM комплексом.
(B) Комплекс ID2 моноубиквитинируется и связывается с зоной повреждения ДНК. Комплекс B-L-100 способствует этому убиквитинированию совместно с еще двумя субкомплексами (A-G-20 and C-E-F)
(C) Нуклеазы XPF/FANCQ-ERCC1 совместно с SLX4/FANCP, incise the Dпроводят ДНК-инцизию
(D) Моноубиквитинированный комплекс ID2 привлекает белки репарации, включая BRCA1, BRCA2/FANCD1, FANCJ, PALB2/FANCN, and RAD51C/FANCO.
(E) После успешной репарации комплекс, ID2 деубиквитинизируется USP1-UAF1 , что способствует его высвобождению из хроматина.
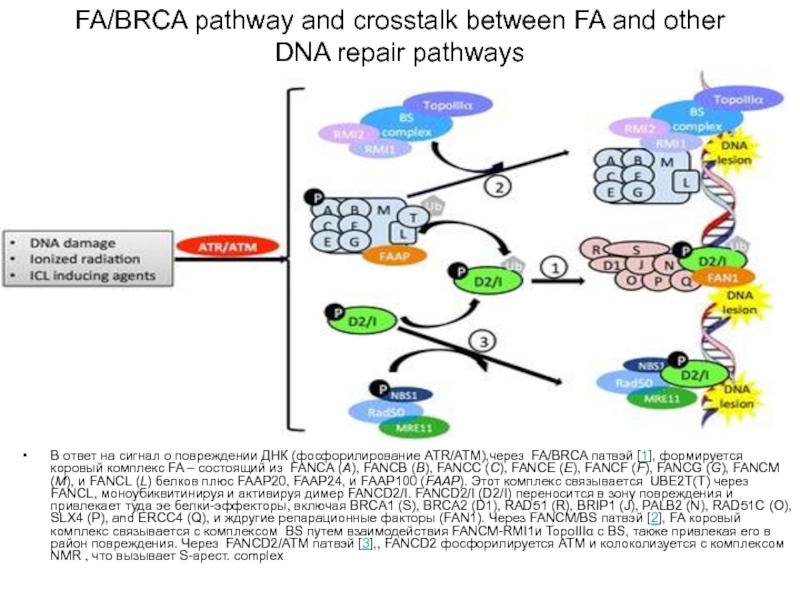
Слайд 77FA/BRCA pathway and crosstalk between FA and other DNA repair pathways
В
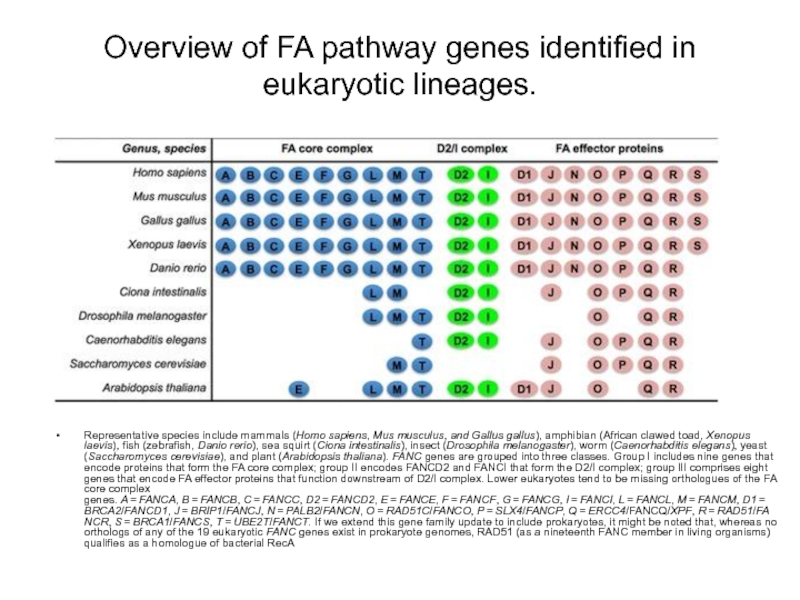
Слайд 78Overview of FA pathway genes identified in eukaryotic lineages.
Representative species include
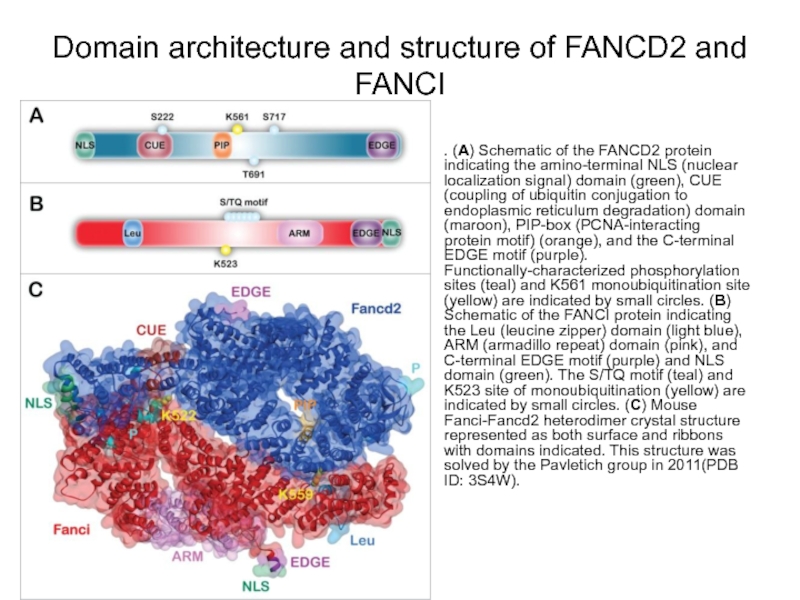
Слайд 79Domain architecture and structure of FANCD2 and FANCI
. (A) Schematic of
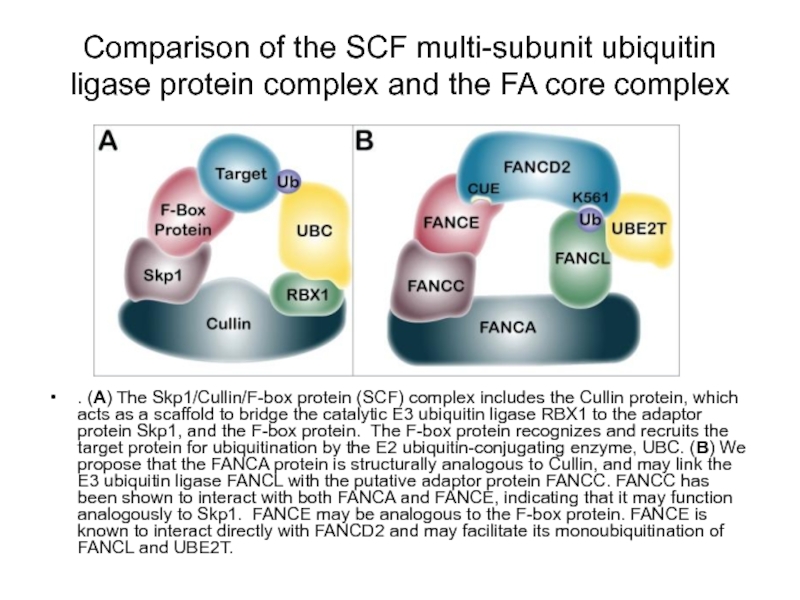
Слайд 80Comparison of the SCF multi-subunit ubiquitin ligase protein complex and the
. (A) The Skp1/Cullin/F-box protein (SCF) complex includes the Cullin protein, which acts as a scaffold to bridge the catalytic E3 ubiquitin ligase RBX1 to the adaptor protein Skp1, and the F-box protein. The F-box protein recognizes and recruits the target protein for ubiquitination by the E2 ubiquitin-conjugating enzyme, UBC. (B) We propose that the FANCA protein is structurally analogous to Cullin, and may link the E3 ubiquitin ligase FANCL with the putative adaptor protein FANCC. FANCC has been shown to interact with both FANCA and FANCE, indicating that it may function analogously to Skp1. FANCE may be analogous to the F-box protein. FANCE is known to interact directly with FANCD2 and may facilitate its monoubiquitination of FANCL and UBE2T.
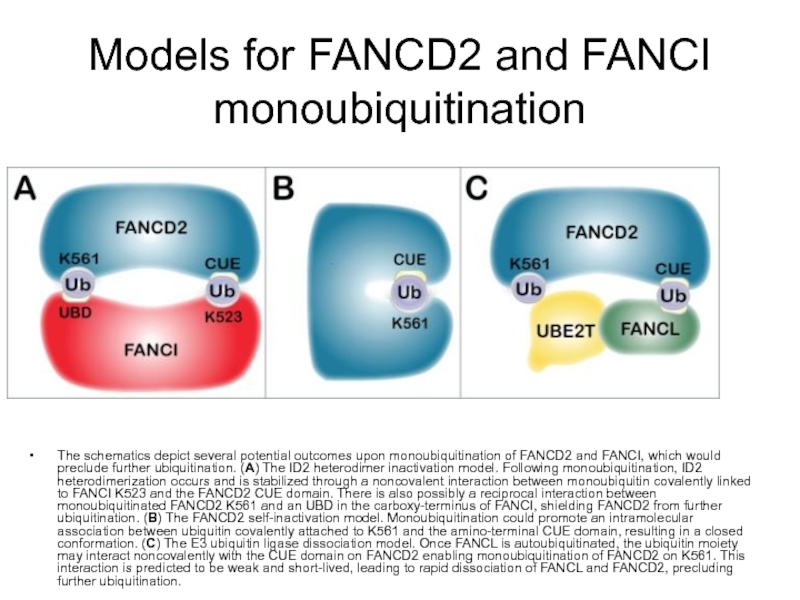
Слайд 81Models for FANCD2 and FANCI monoubiquitination
The schematics depict several potential outcomes
Слайд 83Data acquired in the course of study of primary fibroblasts from
It is just this observation which demonstrates that progeria is a deeply pathological aging and its analogy with the natural aging is limited.
Слайд 90Механизмы G2-ареста
АТМ активирует СНК2 через фосфорелирование триптофана в 68 положении, которая
Вторая ветвь G2-чекпойнта опосредуется через ATR/CHK1 активацию. При этом пути одновременно фосфорелируется-выключается белок CDC25А, а также фосфорелируется серин-549 белка Wee1 (пртеинкиназа), что облегчает его связывание с тем же белком 14-3-3σ и приводит к усилению ингибиторной активности киназ по отношению к CDC2(CDK1). Это придает второй ветви большую гибкость в контроле и консолидации G2-ареста.
Слайд 91Механизмы G2-ареста(2)
После облучения резко падает уровень мРНК циклина В, возможно из-за
Циклин В во время G1 и S фаз имеет цитоплазматическую локализацию и перемещается в ядро только к началу митоза. Белок 14-3-3σ приводит к секвестрированию циклина В в цитоплазме в ответ на повреждение ДНК.
Слайд 92Механизмы G2-ареста(3)
PLK1 и PLK3 (Polo-like kinase). Белки этого семейства принимают активное
Слайд 93Механизмы G2-ареста(4)
Остановить вход в митоз при наличии повреждений в ДНК может
Слайд 94Механизмы G2-ареста(5)
Роль Р53 в поддержании G2-ареста состоит в том, что он
Есть данные о вовлеченности в G2-арест BRCA1, опосредованно через ATM/ATR или напрямую, через активацию CHK1, но механизм этого остается неясным.
G2-реакция клетки на облучение зависит от фазы цикла, в которую это произошло.
Слайд 95Механизмы G2-ареста(6)
G2 чекпойнт-ответ разделяется на два различных пути. Один начинается сразу
Второй, который развивается позже, в клетках, облученных на более ранних стадиях клеточного цикла, является АТМ-независимым, зато зависимым от дозы и приводит к накоплению клеток в фазе G2.
Слайд 96Репарация, спаренная с транскрипцией. TTD
Повышенная фоточувствительность ДНК (примерно в 50% случаев
Нехватка серы в белках волос и их луковиц, ведущая к ломкости волос, "тигровость" волос (чередование светлых и темных полос по длине волоса, выявляемое под микроскопом); ихтиоз; часто умственная и физическая отсталость; дефекты полового развития; аномалии кожи и зубов; нередки раковые заболевания