- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
How to comply with Malaysia Medical Device Regulations 2012? Mourad Kholti 5th March 2014 The 17th SE-Asian Healthcare Show, KLCC, Kuala Lumpur презентация
Содержание
- 1. How to comply with Malaysia Medical Device Regulations 2012? Mourad Kholti 5th March 2014 The 17th SE-Asian Healthcare Show, KLCC, Kuala Lumpur
- 2. Introduction Based in Penang (Malaysia), Andaman Medical
- 3. Contents Definitions Local Authorized representative Arrangement of
- 4. Medical Device Authority (MDA) The Medical Device
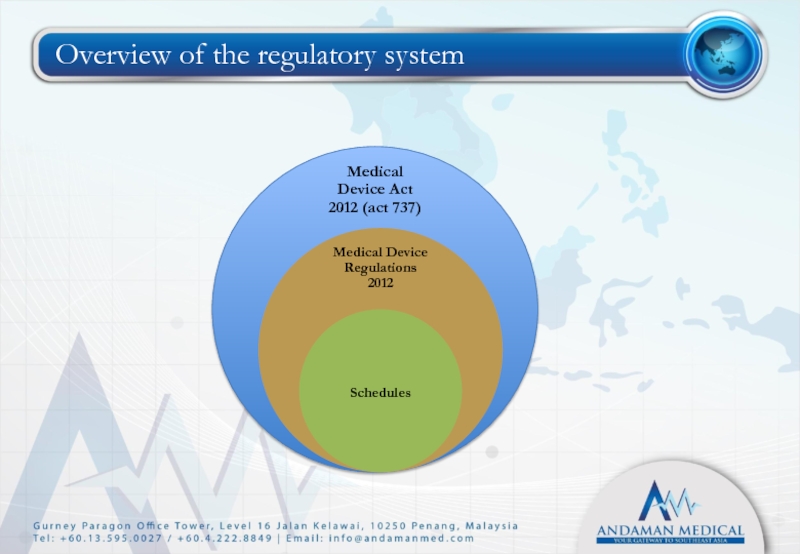
- 5. Overview of the regulatory system
- 6. Contents Definitions Arrangement of the MDR 2012 Schedules Summary Q&A Local Authorized representative
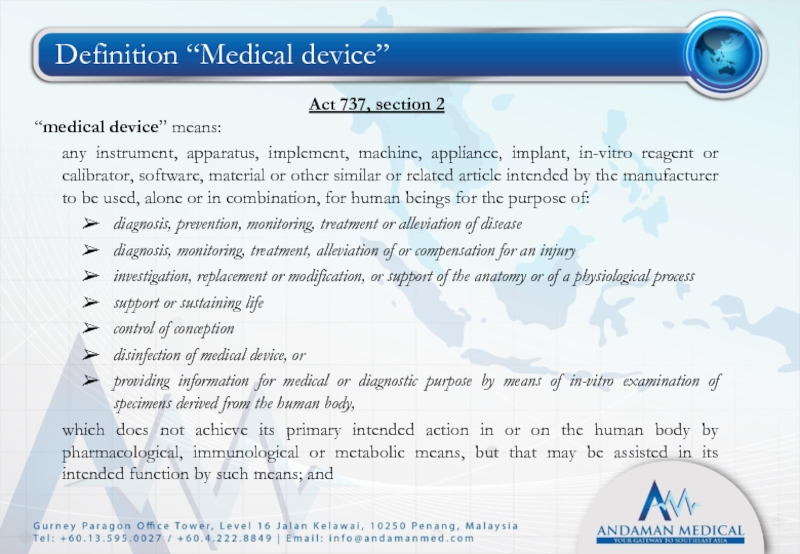
- 7. Definition “Medical device” Act 737, section 2
- 8. Definition “Medical device” b) any instrument, apparatus,
- 9. Definition “Medical device” The compression therapy knee
- 10. Definition “Establishment” Act 737, section 2 “establishment”
- 11. Appointment of LAr Distributor vs Independent
- 12. Contents Definitions Arrangement of the MDR 2012 Schedules Summary Q&A Local Authorized representative
- 13. Medical Device Regulations 2012 Part I: Preliminary
- 14. Part I: Preliminary Regulations come into operation
- 15. Part II: Conformity Assessment procedure All medical
- 16. Part III: Registration of Medical Device Any
- 17. Part IV: Registration of a CAB Any
- 18. Part V: Establishment license Any application for
- 19. Part VII: Labelling requirements Manufacturers shall ensure
- 20. Contents Definitions Arrangement of the MDR 2012 Schedules Summary Q&A Local Authorized representative
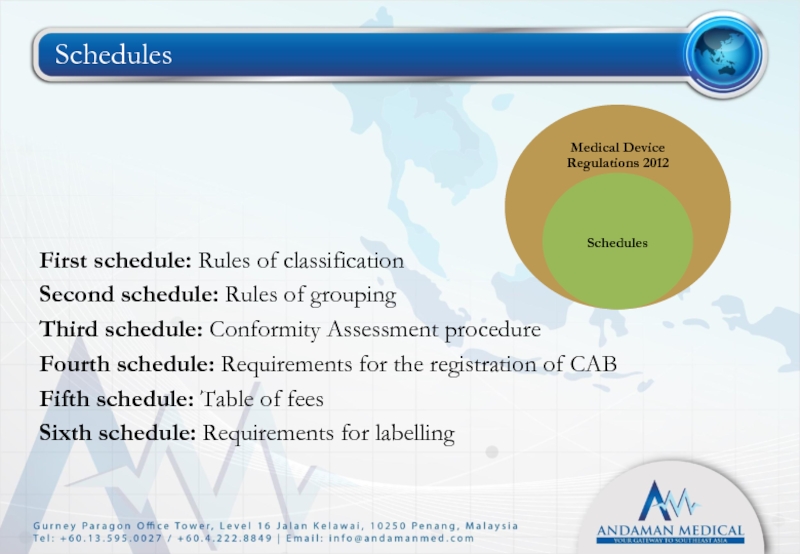
- 21. Schedules First schedule: Rules of classification
- 22. 1st Schedule: Rules of classification Some important
- 23. 1st Schedule: Rules of classification 4
- 24. 1st Schedule: Rules of classification Classification
- 25. 2nd Schedule: Rules of grouping Medical
- 26. 3rd Schedule: Conformity Assessment procedure Collection
- 27. 3rd Schedule: Conformity Assessment procedure Elements
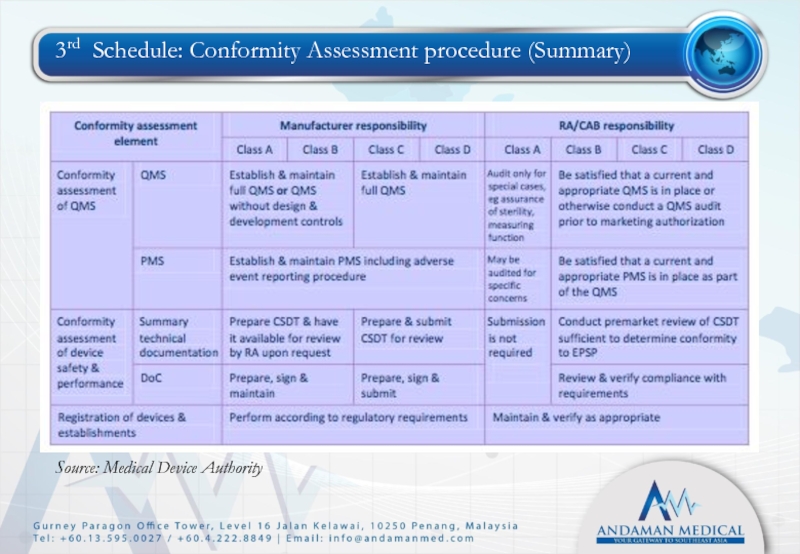
- 28. 3rd Schedule: Conformity Assessment procedure (Summary) Source: Medical Device Authority
- 29. 3rd Schedule: Conformity Assessment procedure (Appendices)
- 30. 4th Schedule: Requirements for the registration of
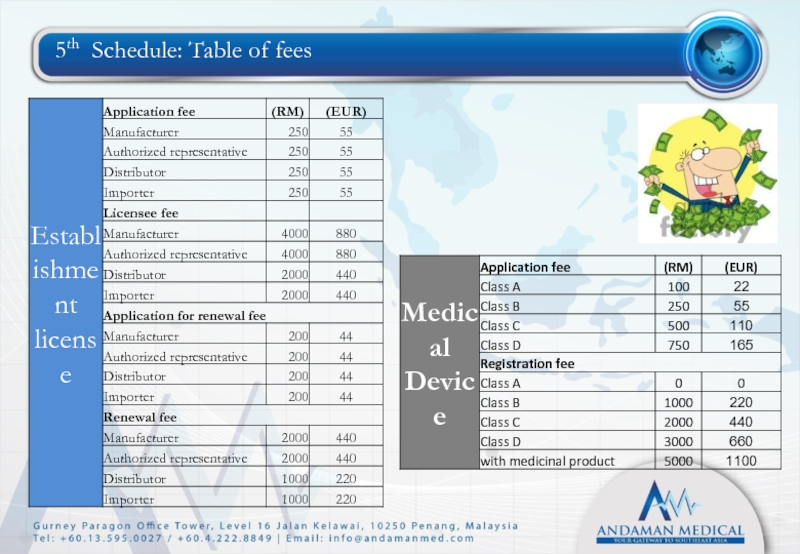
- 31. 5th Schedule: Table of fees
- 32. 6th Schedule: Requirements for labelling Provides
- 33. How do you feel now ?
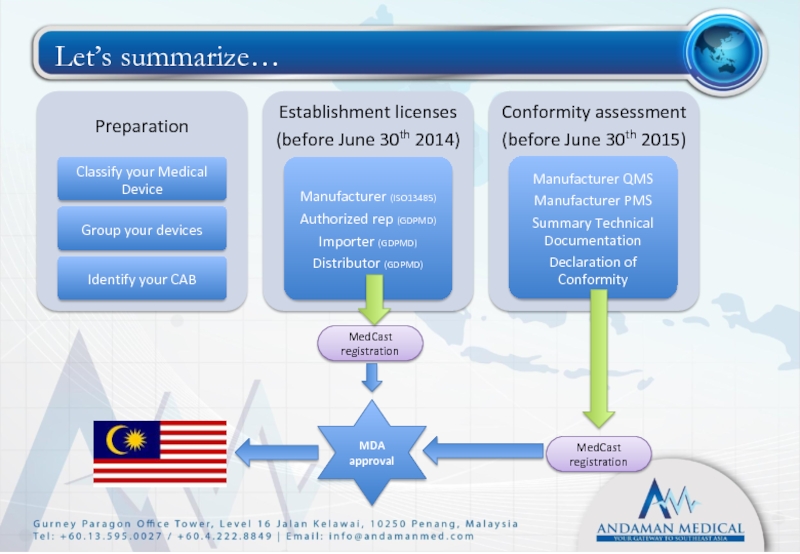
- 34. Let’s summarize… Preparation Classify your Medical
- 35. www.andamanmed.com info@andamanmed.com
- 36. Sources http://www.mdb.gov.my/mdb/
- 37. Q&A
Слайд 1How to comply with
Malaysia Medical Device
Regulations 2012?
Mourad Kholti
5th March 2014
The 17th
KLCC, Kuala Lumpur
Слайд 2Introduction
Based in Penang (Malaysia), Andaman Medical Sdn. Bhd. is a
consulting firm
and Quality Management.
Expertise:
Product registrations & licensing in ASEAN region
Regulatory Strategies in ASEAN and Europe
Quality Management Systems:
(ISO 13485, US FDA 21 CFR part 820, GDPMD)
Clinical Evaluation
Suppliers Auditing
Local Authorized Representation
Certification audit in partnership with a European Notified Body
Слайд 3Contents
Definitions
Local Authorized representative
Arrangement of the MDR 2012
Schedules of MDR 2012
Summary
Q&A
Слайд 4Medical Device Authority (MDA)
The Medical Device Authority (MDA) is a division in the Ministry
Objectives:
to protect the public health and safety and,
to ensure that new technology is made available for use for patients in a timely manner and at the same time facilitating trades and the medical device industry.
How?
through a comprehensive regulatory control and licensing system of:
medical device products
manufacturers, LAR, importers, and distributors.
Слайд 6Contents
Definitions
Arrangement of the MDR 2012
Schedules
Summary
Q&A
Local Authorized representative
Слайд 7Definition “Medical device”
Act 737, section 2
“medical device” means:
any instrument, apparatus, implement,
diagnosis, prevention, monitoring, treatment or alleviation of disease
diagnosis, monitoring, treatment, alleviation of or compensation for an injury
investigation, replacement or modification, or support of the anatomy or of a physiological process
support or sustaining life
control of conception
disinfection of medical device, or
providing information for medical or diagnostic purpose by means of in-vitro examination of specimens derived from the human body,
which does not achieve its primary intended action in or on the human body by pharmacological, immunological or metabolic means, but that may be assisted in its intended function by such means; and
Слайд 8Definition “Medical device”
b) any instrument, apparatus, implement, machine, appliance, implant, in-vitro
Слайд 9Definition “Medical device”
The compression therapy knee brace is indicated for relief
Medical device definition:
…diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap,
Is my product a Medical Device ?
Слайд 10Definition “Establishment”
Act 737, section 2
“establishment” means:
a person who is either a
B) an authorized representative* appointed by a manufacturer having a principal place of business outside Malaysia.
* person domiciled or resident in Malaysia/a firm or company constituted under the laws of Malaysia
Слайд 11Appointment of LAr
Distributor vs Independent Authorized Representative:
Should my distributor focus on
Do I have to scrap all my labels, inserts and packaging, if I want to change distributors ?
In case of incident due to transportation from the distributor to the end-user, will my distributor defend his company or mine?
If my distributor is unable to answer MDA’s questions, does anyone in my company have the skills to do so directly?
Will confidentiality be maintained when sharing a CSDT containing technical information with my distributor?
Will my distributor stay up to date on regulatory changes in Malaysia and will provide me with timely warning when changes affect my devices?
Слайд 12Contents
Definitions
Arrangement of the MDR 2012
Schedules
Summary
Q&A
Local Authorized representative
Слайд 13Medical Device Regulations 2012
Part I: Preliminary
Part II: Conformity Assessment procedure
Part III:
Part IV: Registration of Conformity Assessment Body
Part V: Establishment License
Part VI: Export permit
Part VII: Labelling Requirements
Part VIII: Appeal
Part IX: Register
Слайд 14Part I: Preliminary
Regulations come into operation on 1st July 2013
Provides a
Слайд 15Part II: Conformity Assessment procedure
All medical devices shall be appropriately classified
All medical devices shall be subjected to conformity assessment
Depending on the class, the manufacturer shall appoint a CAB to conduct the assessment
If the conformity assessment is successful, CAB will issue:
Report
Certificate of Conformity
Слайд 16Part III: Registration of Medical Device
Any application for registration of Medical
The application shall be accompanied with:
Application fee
Supporting documents
Any other information required by MDA
Samples of medical device (if required)
If MDA is satisfied, the MD will be kept in the register for a period of 5 years
Cancellation of registration
Слайд 17Part IV: Registration of a CAB
Any person who wants to become
The CAB application shall be accompanied with:
Application fee
Supporting documents
Any other information required by MDA
If MDA is satisfied, the CAB will be registered for a period of
3 years
Слайд 18Part V: Establishment license
Any application for an establishment license shall be
The application shall be accompanied with:
Application fee
Supporting documents
Certificate and report of conformity assessment
Any other information required by MDA
If MDA is satisfied, the establishment license will be issued for a period of 3 years
Suspension or revocation of establishment license
Слайд 19Part VII: Labelling requirements
Manufacturers shall ensure that the MD is appropriately
Shall be legible, permanent, and prominent
No statement saying that the placement in the market is promoted or endorsed by MDA (fine RM10K and/or 3 months imprisonment)
Слайд 20Contents
Definitions
Arrangement of the MDR 2012
Schedules
Summary
Q&A
Local Authorized representative
Слайд 21Schedules
First schedule: Rules of classification
Second schedule: Rules of grouping
Third
Fourth schedule: Requirements for the registration of CAB
Fifth schedule: Table of fees
Sixth schedule: Requirements for labelling
Слайд 221st Schedule: Rules of classification
Some important definitions:
Invasive medical device
Central nervous system
Active
Hazard
Risk
etc…
Слайд 231st Schedule: Rules of classification
4 classes:
The manufacturer shall be responsible for
Слайд 241st Schedule: Rules of classification
Classification rules are provided in 2 appendices
Appendix 1 to classify Medical Devices excluding IVD
Appendix 2 to classify IVD devices
Example: all surgically invasive MD intended for short-term use are in class B (rule 7)
If more than one rule is applicable, the higher classification shall apply.
Слайд 252nd Schedule: Rules of grouping
Medical Devices may be grouped into one
single
family
system
set
in-vitro kit
in-vitro cluster
The basic rules of grouping consist of:
one generic proprietary name
one manufacturer
one common intended purpose
Слайд 263rd Schedule: Conformity Assessment procedure
Collection of evidence of conformity by the
If the manufacturer is not in Malaysia, it shall:
authorize a LAr to act on its behalf
provide all the evidence of conformity
provide necessary support to the LAr for the purpose of the assessment
Depending on the class, a CAB shall be appointed to conduct the assessment.
Слайд 273rd Schedule: Conformity Assessment procedure
Elements of the Conformity assessment:
conformity assessment of
conformity assessment of PMS
conformity assessment of Technical documentation (CSDT)
DoC
Once assessment completed
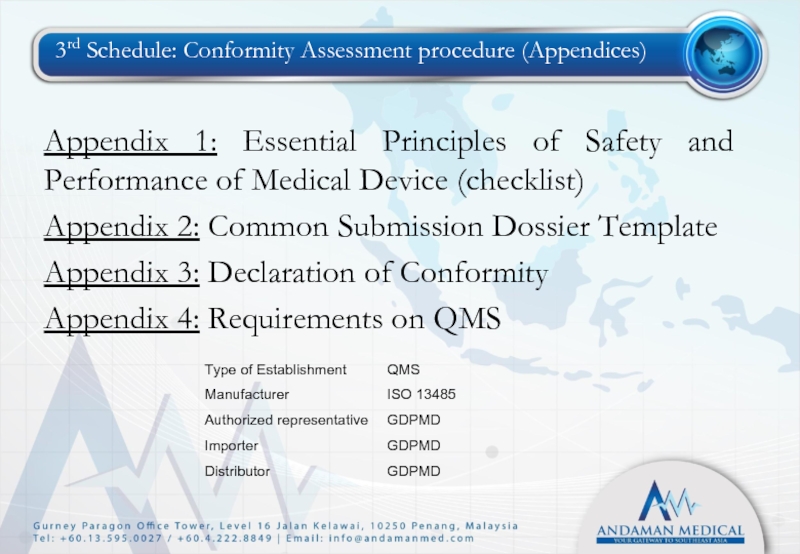
Слайд 293rd Schedule: Conformity Assessment procedure (Appendices)
Appendix 1: Essential Principles of Safety
Appendix 2: Common Submission Dossier Template
Appendix 3: Declaration of Conformity
Appendix 4: Requirements on QMS
Слайд 304th Schedule: Requirements for the registration of CAB’s
This schedule provides the
requirements on organization
requirements on resources and technical competency
requirements on independence and impartiality
requirements on Quality Management System
Слайд 326th Schedule: Requirements for labelling
Provides requirements for:
Location: on the device itself
Format:
Language: Bahasa Melayu for home-used devices (also for others if required by MDA)
Contents: manufacturer AND LAr names, lot nbr, serial nbr, expiry date…
IFU: precautions and warnings, etc…
Слайд 34Let’s summarize…
Preparation
Classify your Medical Device
Group your devices
Identify your CAB
Establishment licenses
(before June
Manufacturer (ISO13485)
Authorized rep (GDPMD)
Importer (GDPMD)
Distributor (GDPMD)
Conformity assessment
(before June 30th 2015)
Manufacturer QMS
Manufacturer PMS
Summary Technical Documentation
Declaration of Conformity
MedCast registration
MedCast registration
MDA approval