- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
I QUIZ-IIISCIENCE Q25.05.2014,NGO QUARTERS HIGHER SECONADARY SCHOOL ,CALICUT презентация
Содержание
- 1. I QUIZ-IIISCIENCE Q25.05.2014,NGO QUARTERS HIGHER SECONADARY SCHOOL ,CALICUT
- 2. ROUND I Infinite pounce/+10 -10
- 3. What does this sequence indicate 2,60,40,1,30,30,5,1,30,200,8,40,50,1, 30,200,8,10,40
- 4. Baa = 2; Seen = 60; Meem
- 5. 5/25/2014
- 6. 5/25/2014
- 7. This is used to check a machine's
- 8. 5/25/2014
- 9. Turing Test 5/25/2014
- 10. These are the
- 11. Magdeberg hemisphere experiment & Levi strauss
- 14. First use of this term in its
- 15. Serendipity
- 16. This symbol is known as Ourobus and
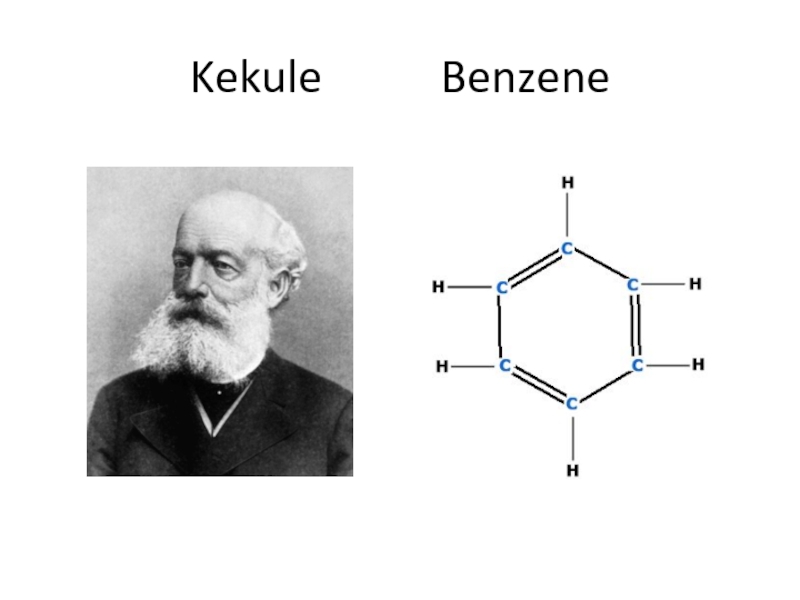
- 17. Kekule Benzene
- 18. Description about a scientific establishment in India
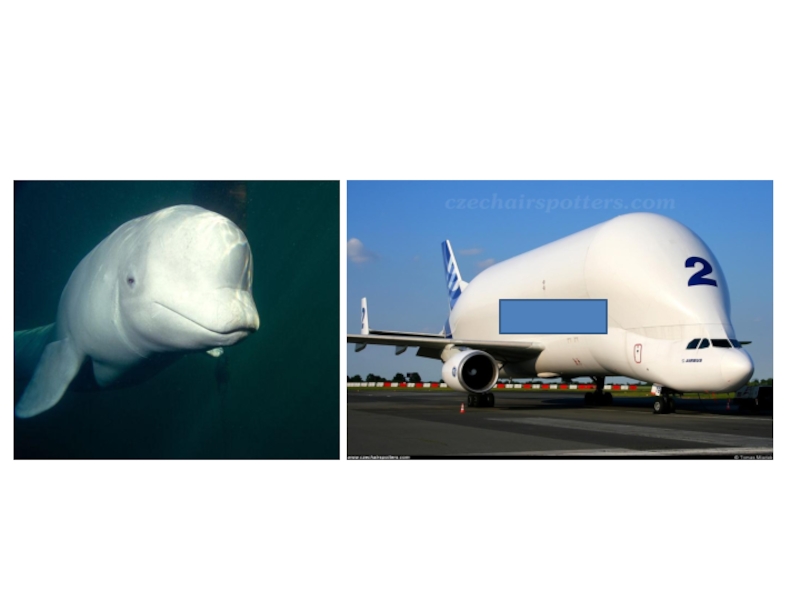
- 21. Beluga Whale & Airbus Beluga
- 22. "Gravity" is a 2013 science fiction
- 23. ISS-International Space Station
- 24. WHO?? Knowledge of Literature – nil. Knowledge
- 26. The name was given by the then
- 27. Apsara,India’s first nuclear reactor
- 29. Programming Languages
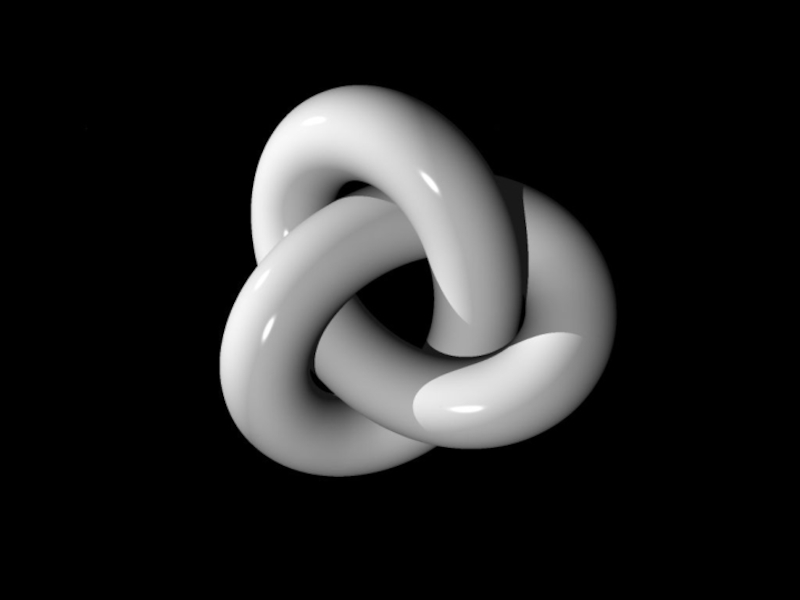
- 30. Knot theory is a branch of topology
- 32. Tangling of headphones/wires inside pockets
- 33. The branding of _____ was created by
- 35. Rheumatoid
- 36. Billy Bowden,& his signalling "crooked finger of doom”
- 37. He was famous during his times as
- 39. Omar Khayyam
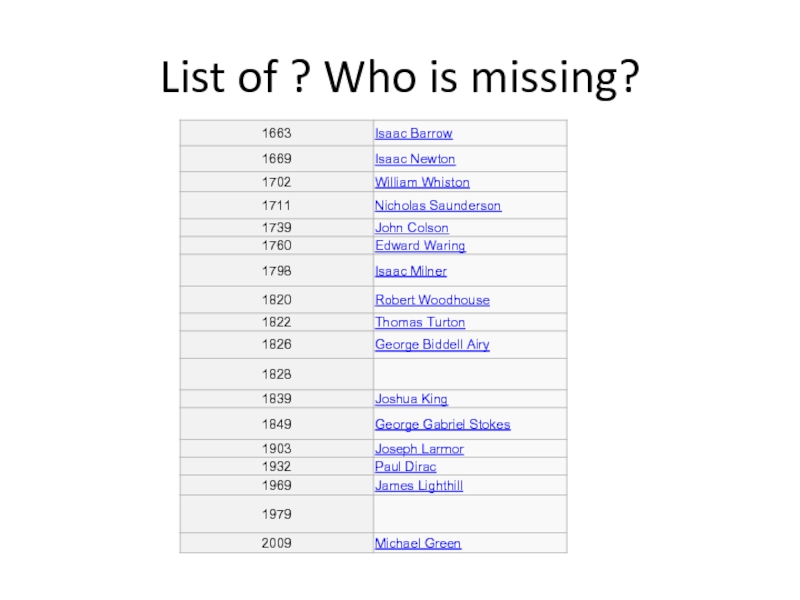
- 40. List of ? Who is missing?
- 41. The Lucasian Chair of Mathematics Missing-Charles babbage, Stephan hawking
- 42. ROUND II Short story!! ABCD? 10 for each-Bonus 10 for all
- 43. ABCD? A was an Indian-American science fiction film in development in
- 44. A-The Alien
- 45. ROUND III Infinite pounce/+10 -10
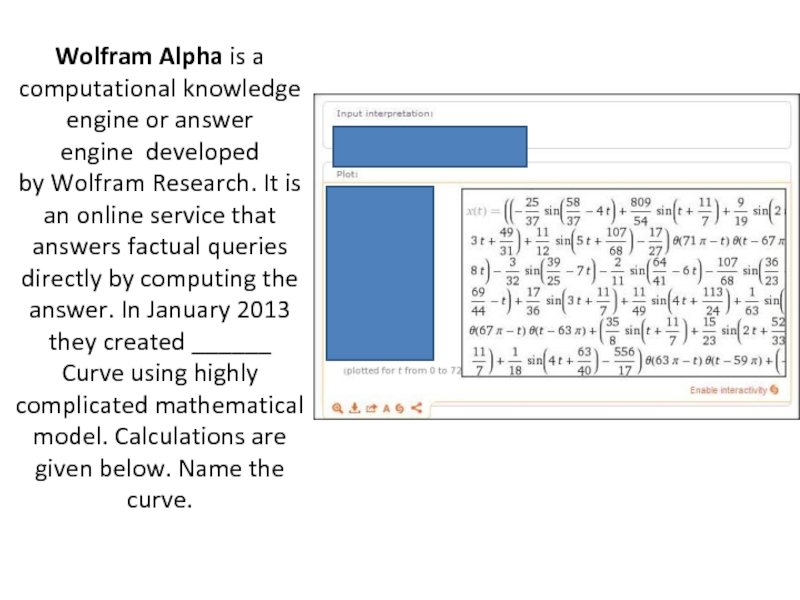
- 46. Wolfram Alpha is a computational knowledge engine or answer
- 49. PSY CURVE
- 50. The large numbers of those who participated
- 52. First IIT in India , IIT Kharagpur
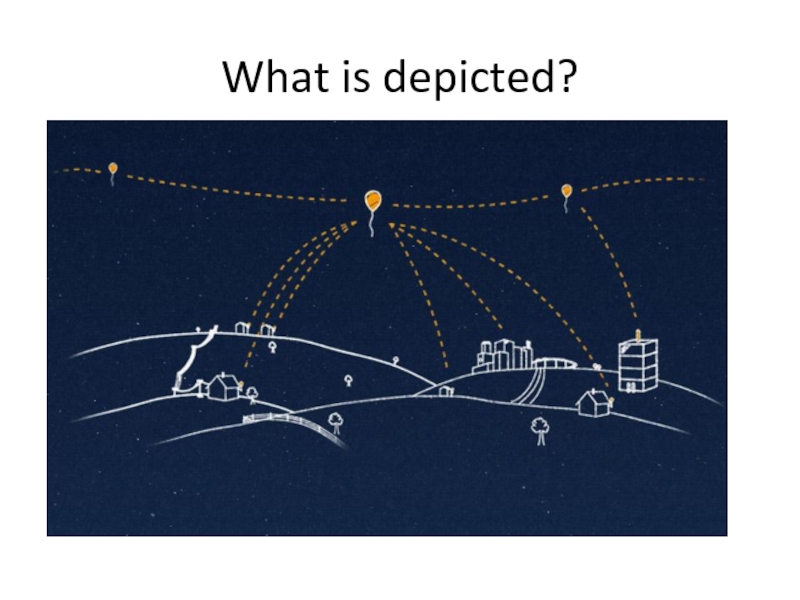
- 55. What is depicted?
- 56. Project Loon is a research and development project being developed
- 59. What does this picture indicates?
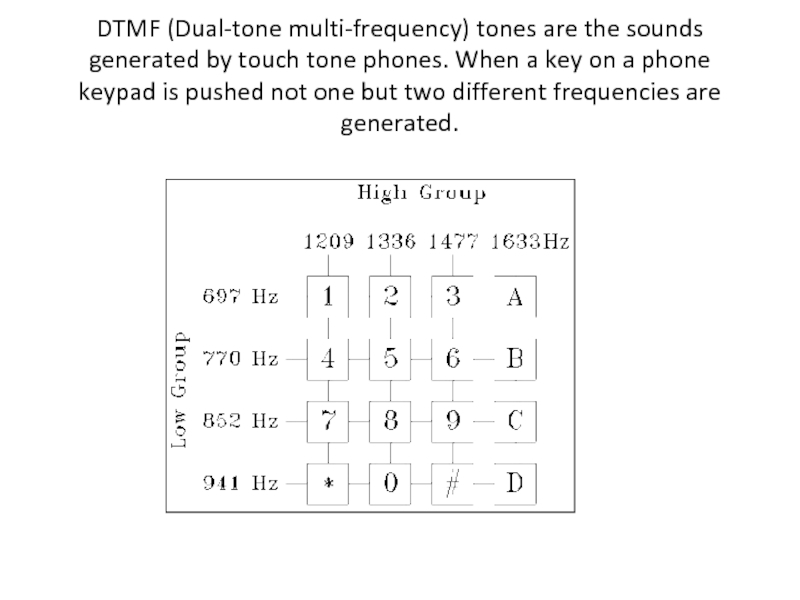
- 60. DTMF (Dual-tone multi-frequency) tones are the sounds
- 61. X is an upcoming biographical film
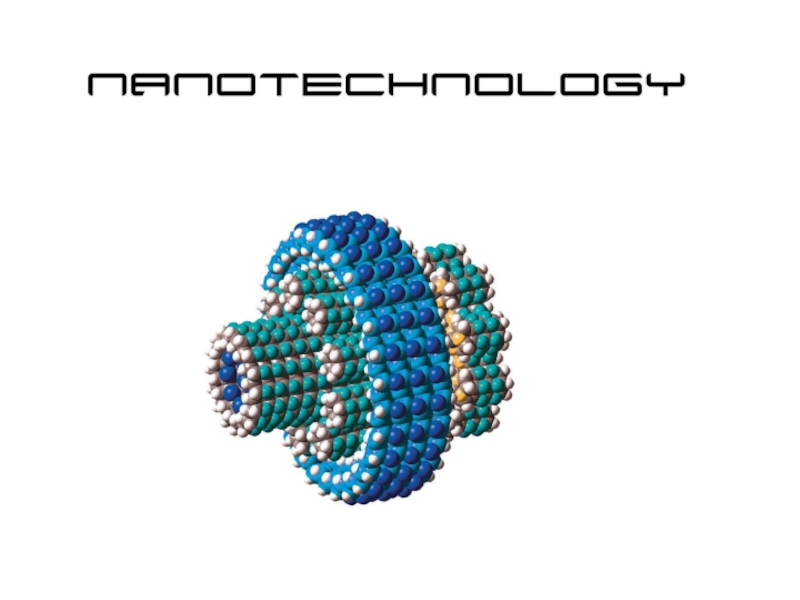
- 63. "There's Plenty of Room at the Bottom" was
- 65. Inspired what? “A is a reduced scale
- 66. Auguste Antoine Piccard inspired Professor calculus in The Adventures of Tintin
- 67. Using a scanning tunneling microscope,Carbon monoxide molecules were manipulated
- 68. a-boy-and-his-atom.mp4
- 69. X is a disorienting neurological
- 70. X- Alice in Wonderland syndrome Y- Lewis Carroll
- 72. Bombay blood group/Hh antigen system
- 74. Kalinga award
- 75. Kind of disease named after a logo 5/25/2014
- 76. Michelin Baby Syndrome 5/25/2014
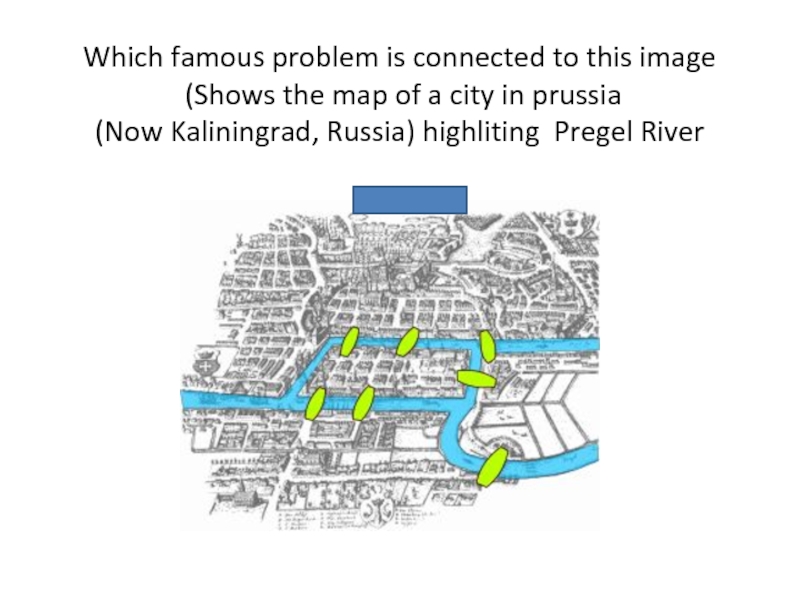
- 77. Which famous problem is connected to
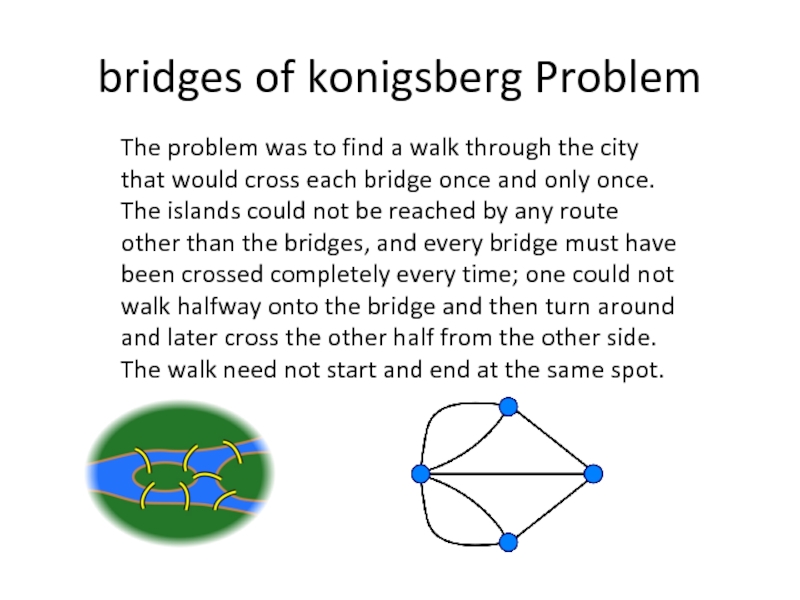
- 78. bridges of konigsberg Problem The problem
- 79. Article from seattle
- 80. Touch screen
- 81. (1) 2 NaN3 → 2
- 82. Deployment of airbag
- 83. X has a career of more than
- 84. Dr. M. Abdul Salam
- 85. ROUND IV THEME 100/-50 to 10/-0
- 86. +100/-50
- 87. +90/-45
- 88. +80/-40
- 89. +70/-35
- 90. +60/-30
- 91. +50/-25
- 92. +40/-20
- 93. +30/-15
- 94. +20
- 95. +10
- 97. Golden Ratio
Слайд 4Baa = 2; Seen = 60; Meem = 40; Alif =
Слайд 7This is used to check a machine's ability to exhibit intelligent
5/25/2014
Слайд 10 These are the original apparatus used for a historic experiment in
Just name the experiment
Which logo was inspired by this Exp
Слайд 14First use of this term in its present meaning was by
Слайд 16This symbol is known as Ourobus and is seen in several
Слайд 18Description about a scientific establishment in India A local Catholic church, the
Слайд 22 "Gravity" is a 2013 science fiction thriller directed bt Alfonso Cuaron
Слайд 24WHO?? Knowledge of Literature – nil. Knowledge of Philosophy – nil. Knowledge of Astronomy
Слайд 26The name was given by the then Prime Minister of India, Jawaharlal
Слайд 30Knot theory is a branch of topology dealing with the study
Слайд 33The branding of _____ was created by Timothy Hanley to distinguish
Слайд 35 Rheumatoid arthritis (RA) is an autoimmune disease that results in a chronic,systemic inflammatory disorder that may
Слайд 37He was famous during his times as a mathematician. He wrote the
Слайд 43ABCD? A was an Indian-American science fiction film in development in the late 1960s which was
Слайд 46Wolfram Alpha is a computational knowledge engine or answer engine developed by Wolfram Research.
Слайд 50The large numbers of those who participated in the armed struggle
Слайд 56Project Loon is a research and development project being developed by Google with the mission
Слайд 60DTMF (Dual-tone multi-frequency) tones are the sounds generated by touch tone
Слайд 61 X is an upcoming biographical film based on the life of renowned scientist.It
Слайд 63"There's Plenty of Room at the Bottom" was a lecture given by physicist Richard
Слайд 65Inspired what? “A is a reduced scale Q, as the real chap
Слайд 67Using a scanning tunneling microscope,Carbon monoxide molecules were manipulated into place on a
Слайд 69 X is a disorienting neurological condition that affects human perception. Sufferers may experience micropsia, macropsia,
Слайд 77 Which famous problem is connected to this image (Shows the map
Слайд 78bridges of konigsberg Problem
The problem was to find a walk through
Слайд 79 Article from seattle times: British researchers provide some stomach-churning data: ______ harbor
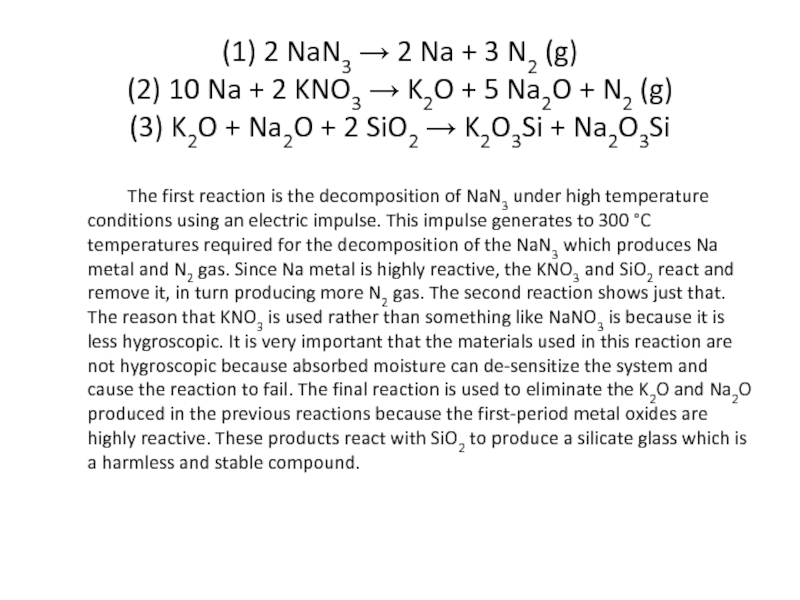
Слайд 81 (1) 2 NaN3 → 2 Na + 3 N2 (g) (2) 10 Na +
The first reaction is the decomposition of NaN3 under high temperature conditions using an electric impulse. This impulse generates to 300 °C temperatures required for the decomposition of the NaN3 which produces Na metal and N2 gas. Since Na metal is highly reactive, the KNO3 and SiO2 react and remove it, in turn producing more N2 gas. The second reaction shows just that. The reason that KNO3 is used rather than something like NaNO3 is because it is less hygroscopic. It is very important that the materials used in this reaction are not hygroscopic because absorbed moisture can de-sensitize the system and cause the reaction to fail. The final reaction is used to eliminate the K2O and Na2O produced in the previous reactions because the first-period metal oxides are highly reactive. These products react with SiO2 to produce a silicate glass which is a harmless and stable compound.
Слайд 83X has a career of more than three decades spanning across