- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Immunophysiology of renal system презентация
Содержание
- 1. Immunophysiology of renal system
- 10. The kidneys purify toxic metabolic waste products
- 11. The kidneys produce several hormones
- 12. Vitamin D regulates the innate and adaptive
- 14. Renal tubular epithelial cells (TECs) play an
- 15. Proximal tubule epithelial cells (PTEC) of the
- 17. Damaged tubule with infiltrating dendritic cells. b | In the
- 19. Resident renal mononuclear phagocytes (rMoPh)
- 20. Apart from their role in the clearance
- 21. The heterogeneous but overlapping phenotype and functions
- 24. Distinct and shared functions of macrophages and dendritic cells in the kidney
- 25. ‘Summary at a glance’: functions of DCs
- 26. major challenges facing the field in the
- 27. Significant progress in understanding the renal mononuclear
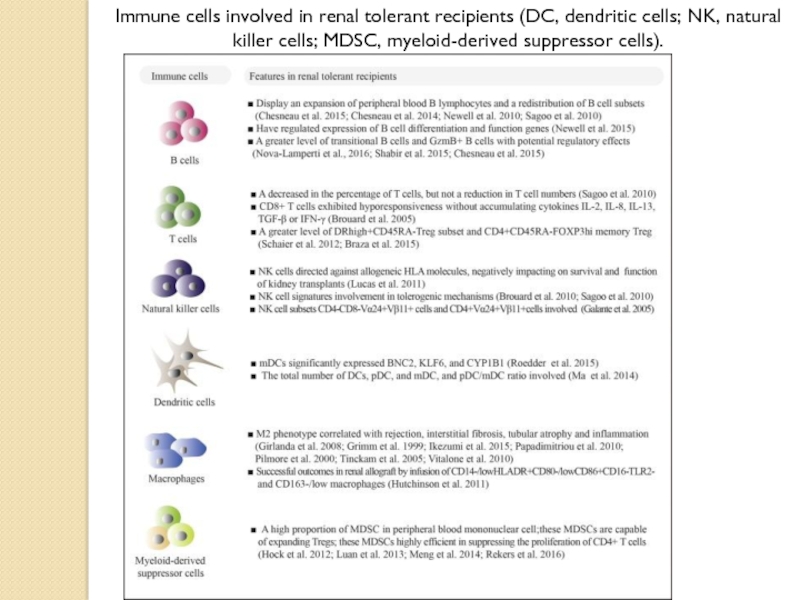
- 28. Immune cells involved in renal tolerant recipients
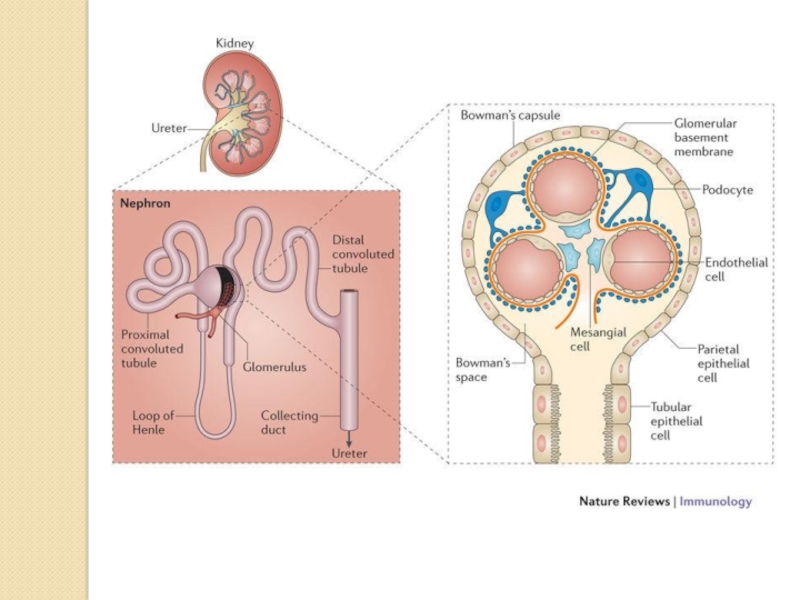
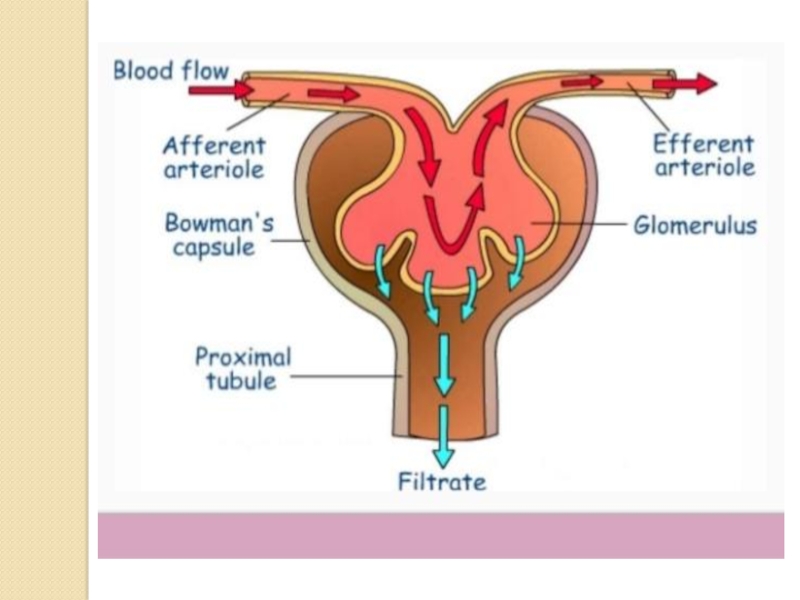
Слайд 10The kidneys purify toxic metabolic waste products from the blood in
Слайд 11
The kidneys produce several hormones that directly or indirectly affect
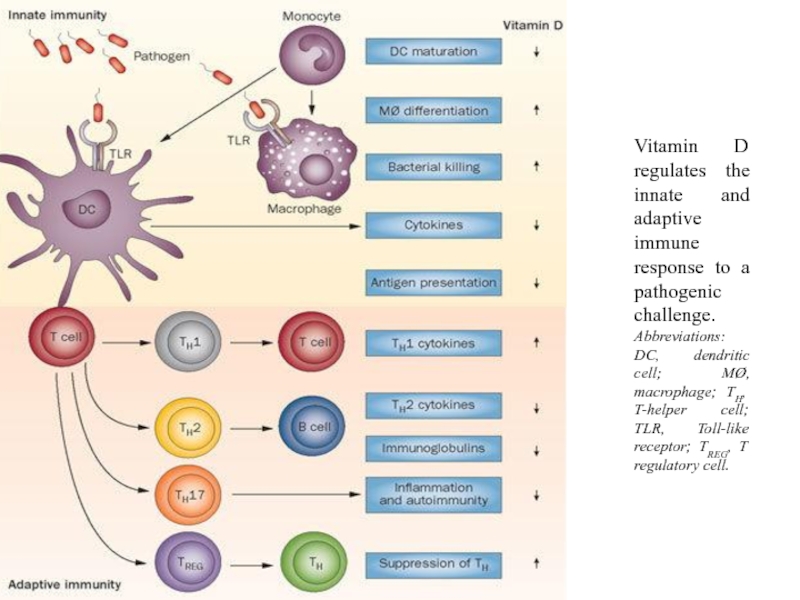
Слайд 12Vitamin D regulates the innate and adaptive immune response to a
Слайд 14Renal tubular epithelial cells (TECs) play an active role in renal
The TECs exert immunosuppressive effects on CD4+ and CD8+ T cell proliferation and lead to enhanced T cell apoptosis. This would mean that, in the renal microenvironment, T cells in contact with the TEC barrier are exposed to more inactivation and death by TECs. Infiltrating T cells in the renal interstitial compartment will still be able to mount effective immune responses against alloantigens.
Moreover, TECs could also induce regulatory CD4+ T cells being able to inhibit the proliferation of other immune cells.
Parenchymal cells have been shown to exert their immunosuppressive effects in a cell–cell contact-dependent manner, as supernatant experiments did not reveal any inhibitory effect.
Слайд 15Proximal tubule epithelial cells (PTEC) of the kidney line the proximal
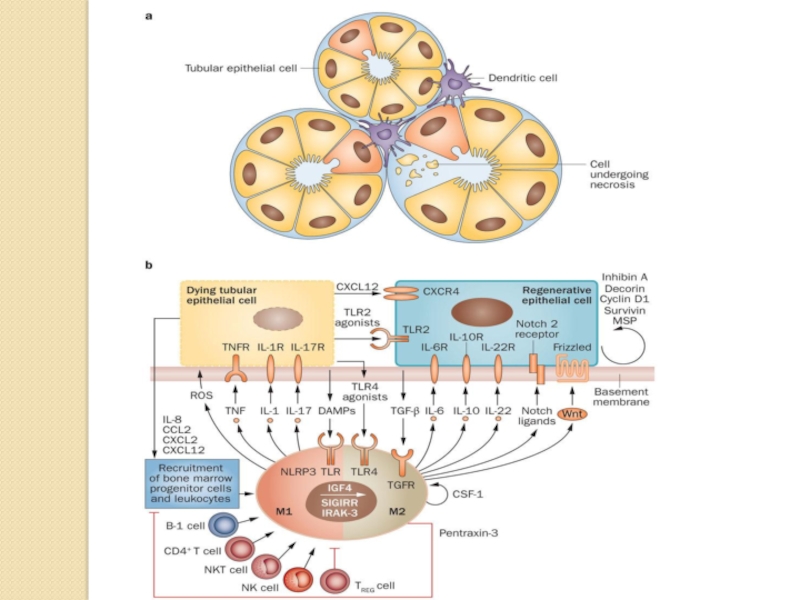
Слайд 17 Damaged tubule with infiltrating dendritic cells. b | In the early phase of acute
Слайд 19Resident renal mononuclear phagocytes (rMoPh)
The ability of rMoPh to change
anti-inflammatory and reparative factors in response to challenge with lipopolysaccharide or ischemic injury, respectively.
Слайд 20Apart from their role in the clearance of dying cells, fetal
The kidney has a remarkable ability to regenerate following acute injury. Most notably, the renal epithelia have the intrinsic capacity to rapidly self-duplicate.
Although the majority of regenerating tubular epithelial cells are derived from an intrarenal source, macrophages cells may contribute to the replacement of tubular epithelial cells through a process of cell fusion, as has been shown in the liver. Macrophages demonstrate cell plasticity and have the ability to undergo cell-cell fusion with themselves or other cell types, particularly in response to inflammatory stimuli. Mature blood monocytes and inflammatory macrophages have been shown to transform into vascular elements including endothelial cells, myofibroblasts, and smooth muscle cells in addition to neuronal and liver cells. It was discovered that cell fusion events occur between renal cells and macrophages or their highly proliferative progenitors.
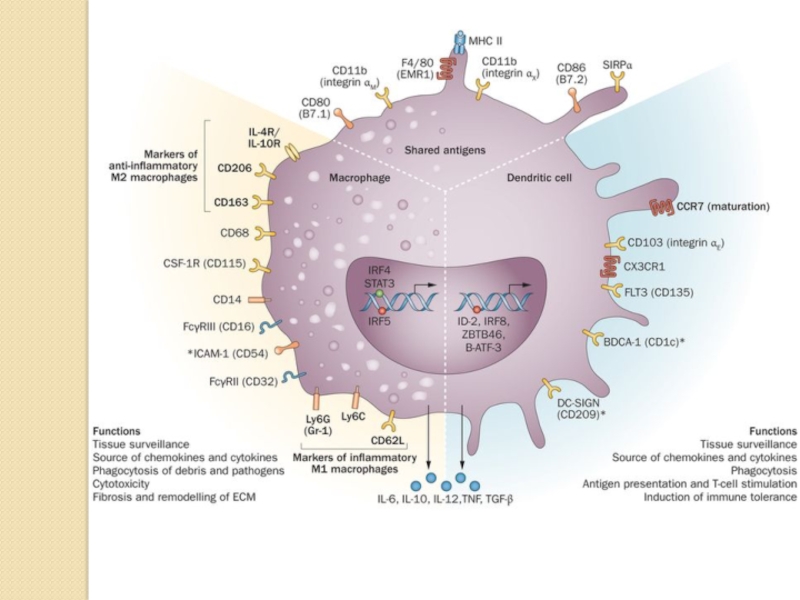
Слайд 21The heterogeneous but overlapping phenotype and functions of renal DCs and
DCs are traditionally described as mediators of immune surveillance and antigen presentation, and as the primary determinants of responses to antigens—through initiation of either immune effector-cell functions or the development of tolerance. Macrophages also function as innate immune cells, predominantly through phagocytosis and production of toxic metabolites. However, the classical paradigm of DC versus macrophage phenotypes and functions is increasingly indistinct within the kidney, as these cells exhibit overlapping surface markers, functional capabilities, and ontogenic pathways. This molecular and phenotypic overlap between cell types and subsets complicates their identification and evaluation.
Renal DCs and macrophages are phenotypically and functionally heterogeneous cells that regulate tissue responses to renal injury and disease. The considerable overlap between DCs and macrophages represents a continuum of phenotype, as well as plasticity of cells of the myeloid–monocytic lineage both in vivo and in vitro.
Under homeostatic conditions, the resident immune cells of the kidneys include dendritic cells (DCs) and macrophages, as well as a few lymphocytes. DCs are restricted to the tubulointerstitium and are absent from the glomeruli. Macrophages are preferentially found in the renal medulla and capsule and have homeostatic and repair functions.
Слайд 25‘Summary at a glance’: functions of DCs
1. Renal dendritic cells (rDCs)
2. rDCs form an extensive surveillance network in the kidney
tubulointerstitium, alerting to infections/injury.
3. rDCs may exacerbate acute non-immune kidney injury (e.g., ischemia
reperfusion injury (IRI) or unilateral ureter obstruction (UUO)) by inducing harmful immune effector mechanisms.
4. rDCs have protective anti-inflammatory roles in acute GN, but may
acquire injurious pro-inflammatory properties in chronic renal
inflammation.
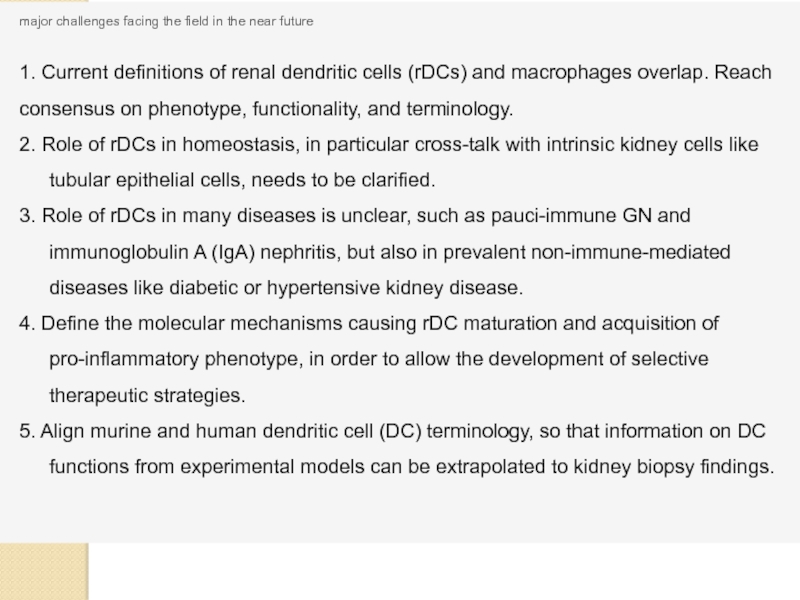
Слайд 26major challenges facing the field in the near future
1. Current definitions
2. Role of rDCs in homeostasis, in particular cross-talk with intrinsic kidney cells like tubular epithelial cells, needs to be clarified.
3. Role of rDCs in many diseases is unclear, such as pauci-immune GN and immunoglobulin A (IgA) nephritis, but also in prevalent non-immune-mediated diseases like diabetic or hypertensive kidney disease.
4. Define the molecular mechanisms causing rDC maturation and acquisition of pro-inflammatory phenotype, in order to allow the development of selective therapeutic strategies.
5. Align murine and human dendritic cell (DC) terminology, so that information on DC functions from experimental models can be extrapolated to kidney biopsy findings.