- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Reactions and equations lab презентация
Содержание
- 1. Reactions and equations lab
- 2. Purpose To observe 7 reactions To identify
- 3. Evidence of Chemical Reaction Part A R1
- 4. Evidence of Chemical Reaction Part C R1
- 5. Evidence of Chemical Reaction Part C R2
- 6. Evidence of Chemical Reaction Part C R3
- 7. Word Equations Part A A1: Magnesium plus
- 8. Word Equations Part B B1: Copper (II)
- 9. Word Equations Part C C1 Iron plus
- 10. Instructions Complete the word equations by predicting
- 11. The End Complete the equations for the
- 12. This powerpoint was kindly donated to www.worldofteaching.com
Слайд 2Purpose
To observe 7 reactions
To identify and write formulas for the reactants
and products of those reactions
To balance the equations for the reactions
To balance the equations for the reactions
Слайд 3Evidence of Chemical Reaction
Part A
R1 Mg ribbon is ash-like now
R2 Litmus
test indicates a base
Part B
R1 Solid turns white, steam comes from test tube
R2 Solid turns blue again
Part B
R1 Solid turns white, steam comes from test tube
R2 Solid turns blue again
Слайд 4Evidence of Chemical Reaction
Part C
R1 Fe nail is now coated in
Cu and the solution has changed from a blue color to a greenish color
Слайд 5Evidence of Chemical Reaction
Part C
R2 Cu metal is now coated with
Ag and the solution is beginning to take on a slightly blue tint
Слайд 6Evidence of Chemical Reaction
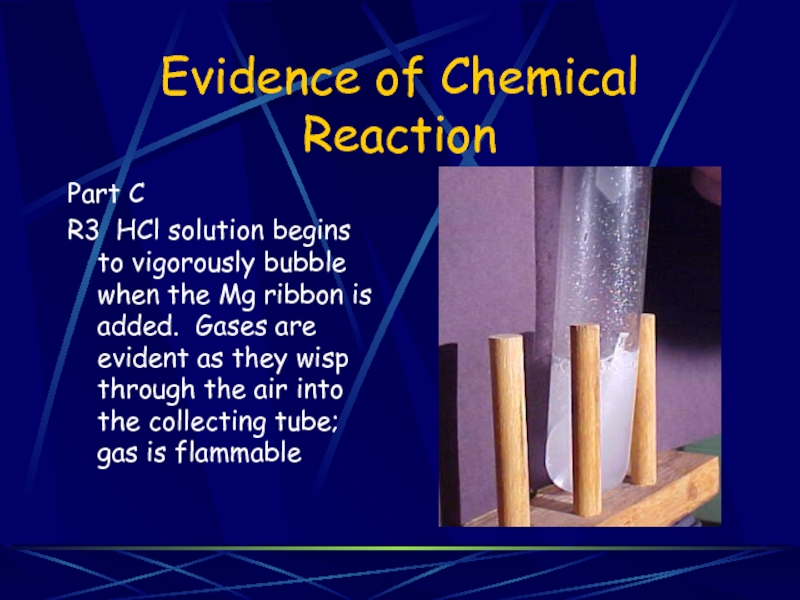
Part C
R3 HCl solution begins to vigorously bubble
when the Mg ribbon is added. Gases are evident as they wisp through the air into the collecting tube; gas is flammable
Слайд 8Word Equations Part B
B1: Copper (II) Sulfate Pentahydrate is heated to
yield ?
B2 Anhydrous Copper (II) Sulfate plus water yields ?
B2 Anhydrous Copper (II) Sulfate plus water yields ?
Слайд 9Word Equations Part C
C1 Iron plus Copper (II) Sulfate yields… ?
C2
Copper plus Silver Nitrate yields… ?
C3 Magnesium plus Hydrochloric Acid yields… ?
C3 Magnesium plus Hydrochloric Acid yields… ?
Слайд 10Instructions
Complete the word equations by predicting the products, use the information
you gathered by observation to help in the predictions
Be sure to include the state of the substance for the reactants and products
Classify the reaction
Be sure to include the state of the substance for the reactants and products
Classify the reaction
Слайд 11The End
Complete the equations for the reactions
Then you may go
finish Part D of the lab instructions. You will need the solubility rules.
Слайд 12This powerpoint was kindly donated to www.worldofteaching.com
http://www.worldofteaching.com is home to over
a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching.