- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Physical chemistry of surface phenomena. Basics of adsorptive therapy презентация
Содержание
- 1. Physical chemistry of surface phenomena. Basics of adsorptive therapy
- 2. SURFACE PHENOMENA are phenomena associated with the
- 3. Surface phenomena Inside the fluid forces are
- 4. Surface phenomena The increased surface area of
- 5. Surface tension σ is the work required
- 6. Surface tension Surface tension depends on:
- 7. SORPTION
- 8. Medical & biological importance: Assimilation
- 9. Sorption -change in the concentration of one
- 10. Adsorption Adsorption is spontaneous change of component
- 11. Gibbs Equation G - the amount of
- 12. Surface activity The ability of the solute
- 13. Traube-Duclos rule: When extending the chain-CH2 -
- 14. SAS, SIS, SNS Surface-active substances (SAS):
- 15. The isotherm of surface tension The dependence
- 16. The structure of SAS molecules: SAS molecule
- 17. ADSORPTION ON THE SOLUTION-GAS BORDER
- 18. Calculation of the adsorption isotherm G=
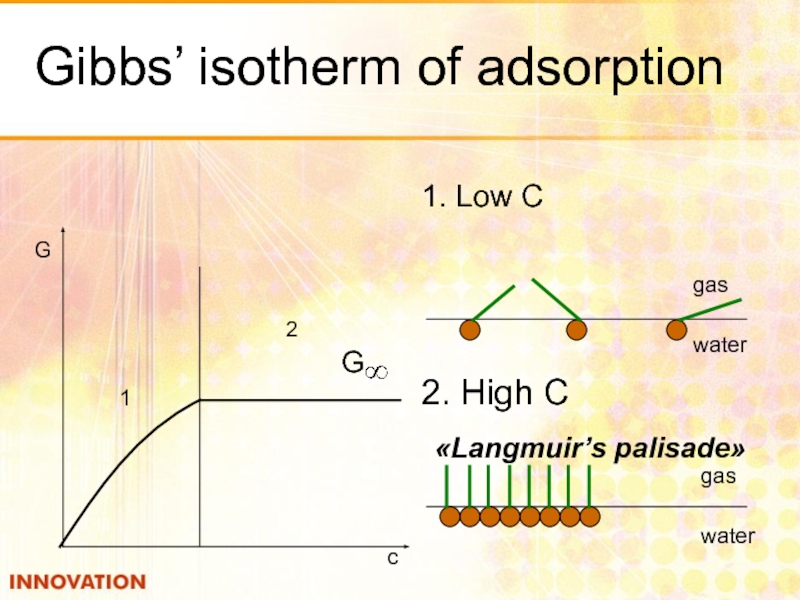
- 19. Gibbs’ isotherm of adsorption 1. Low С
- 20. ADSORPTION ON THE SOLID-GAS BORDER
- 21. Adsorption by solids The adsorption value depends
- 22. Freundlich equation А = x/m = k
- 23. Determination of the constants in the Freundlich
- 24. The theory of Langmuir 1) On each
- 25. The theory of Langmuir According to this
- 26. Langmuir equation А = А
- 27. To find the constants A
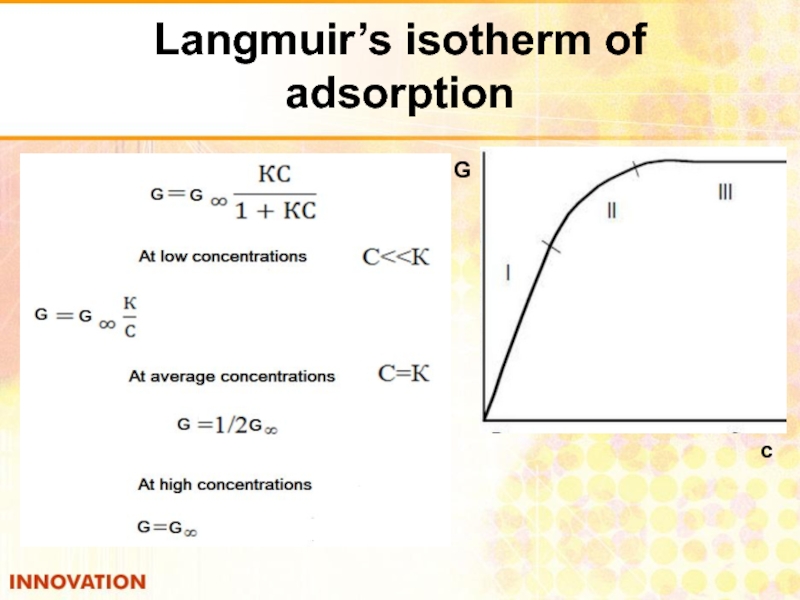
- 28. Langmuir’s isotherm of adsorption с G
- 29. Theory of polymolecular adsorption BET (Brunauer, Emmett,
- 30. ADSORPTION ON THE BORDER OF SOLID
- 31. Molecular adsorption Experimentally determined value of the
- 32. Molecular adsorption So, in the adsorption of
- 33. Conclusion From the above that is confirmed,
- 34. The ion exchange adsorption The ion exchange
- 35. Chromatography Chromatography is dynamic method of analysis
- 36. Chromatography is physical chemical method
- 37. From the history of chromatography Mikhail Semenovich
- 38. «No other discovery had such a huge
- 39. The principle of chromatographic separation of substances
- 40. Column chromatography the stationary phase is in
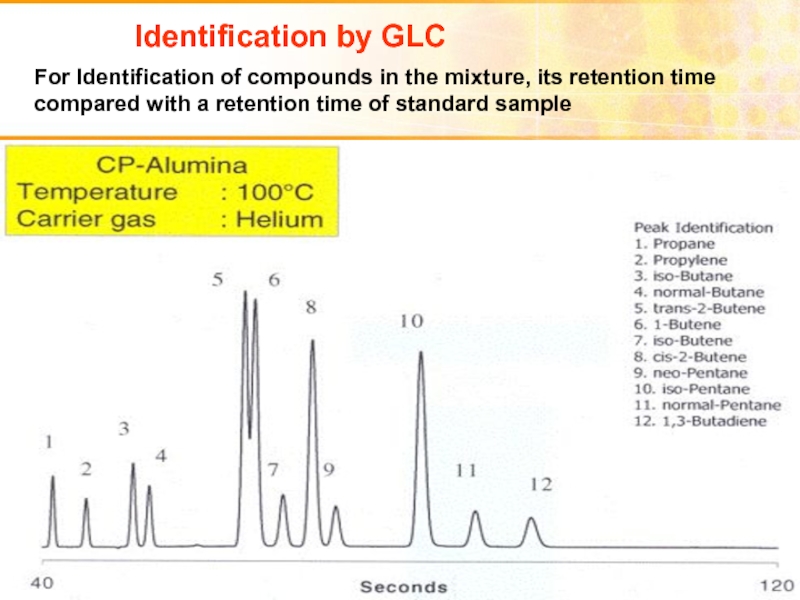
- 41. Identification by GLC For Identification of compounds
- 42. HPLC Agilent Technologies
- 43. HPLC Milichrom
- 44. HPLC HP
- 45. GLC “Agilent Technologies”
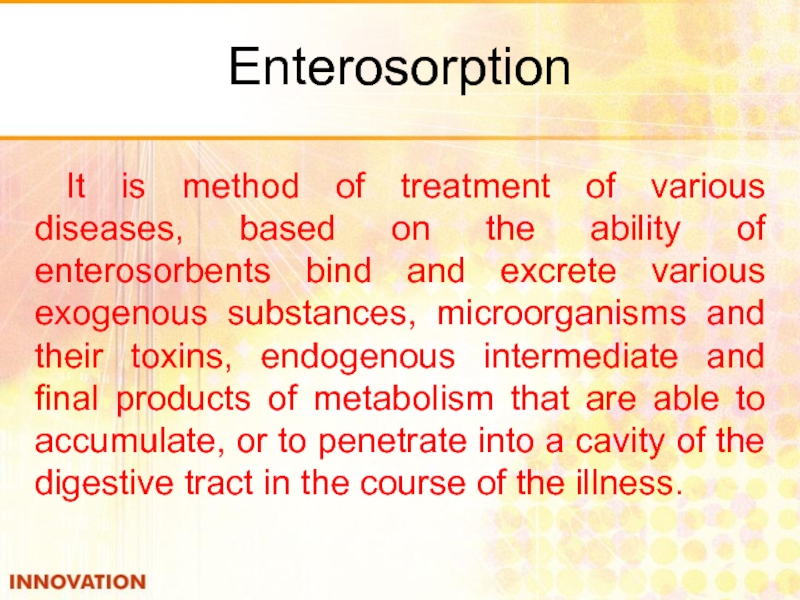
- 46. Enterosorption It is method of treatment of
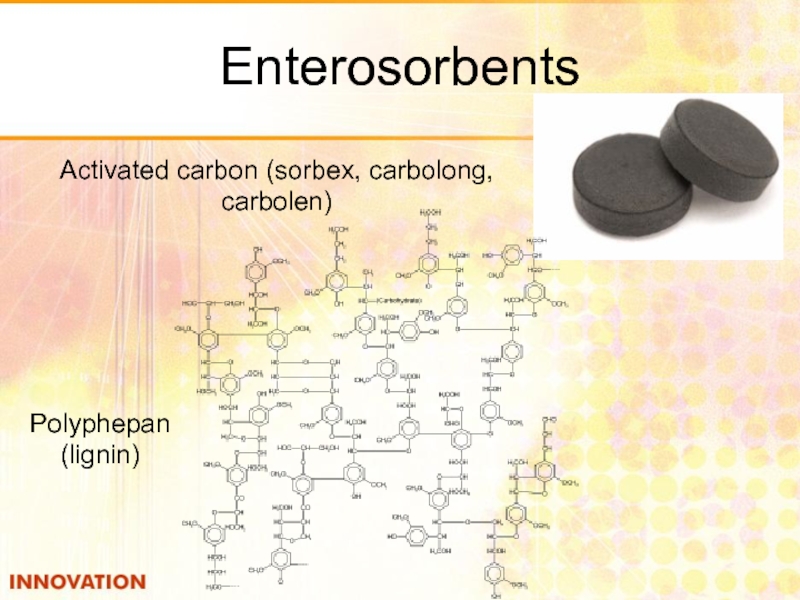
- 47. Enterosorbents Polyphepan (lignin) Activated carbon (sorbex, carbolong, carbolen)
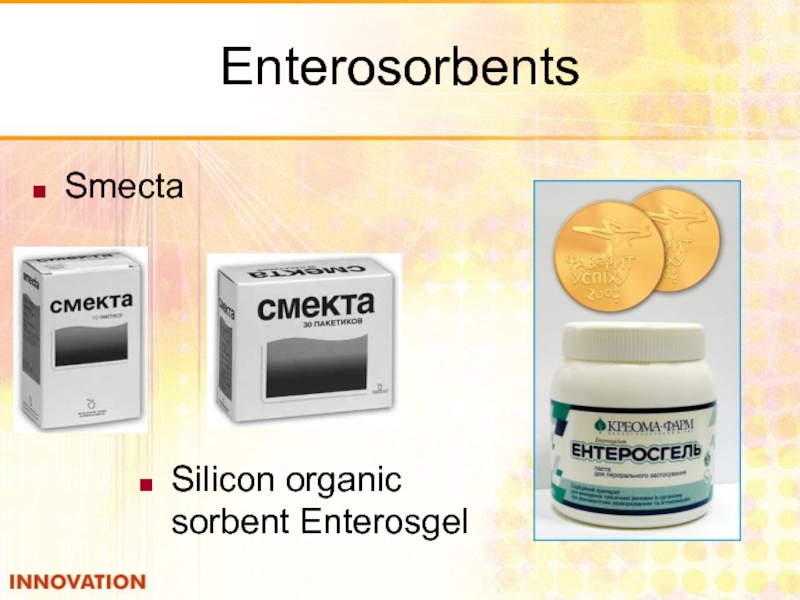
- 48. Enterosorbents Smecta Silicon organic sorbent Enterosgel
- 49. Enterosorption Enterosorption is part of efferent therapy
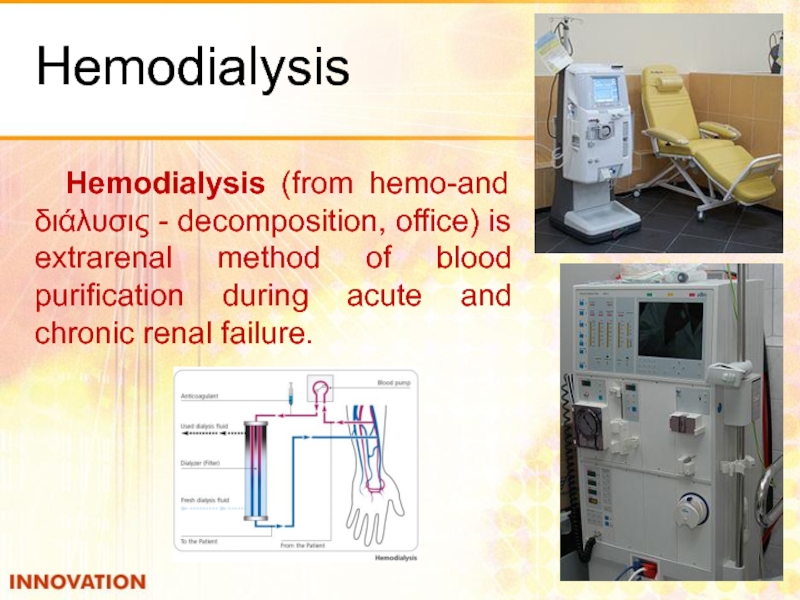
- 50. Hemodialysis Hemodialysis (from hemo-and διάλυσις - decomposition,
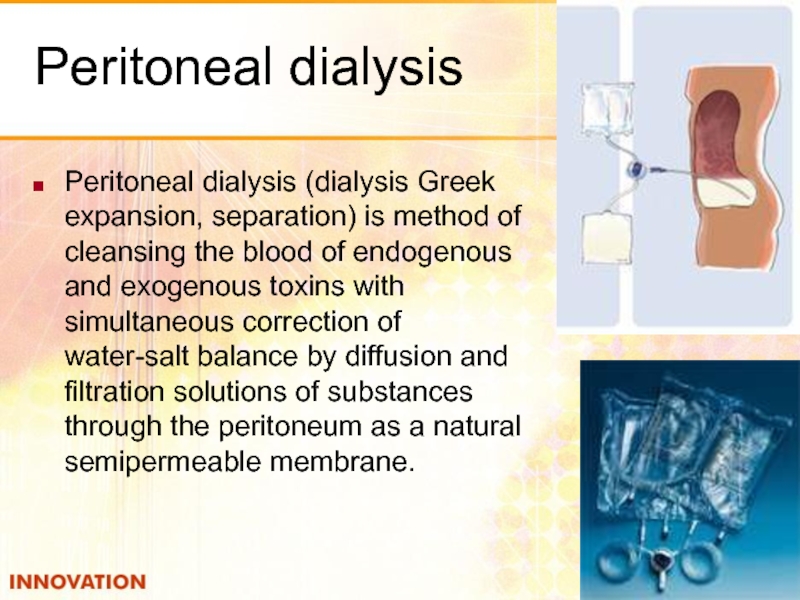
- 51. Peritoneal dialysis Peritoneal dialysis (dialysis Greek expansion,
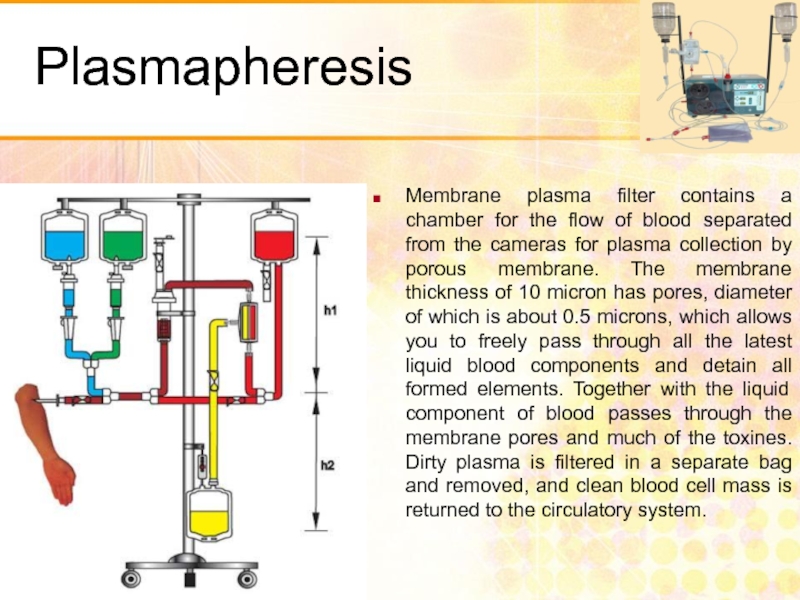
- 52. Plasmapheresis Membrane plasma filter contains a chamber
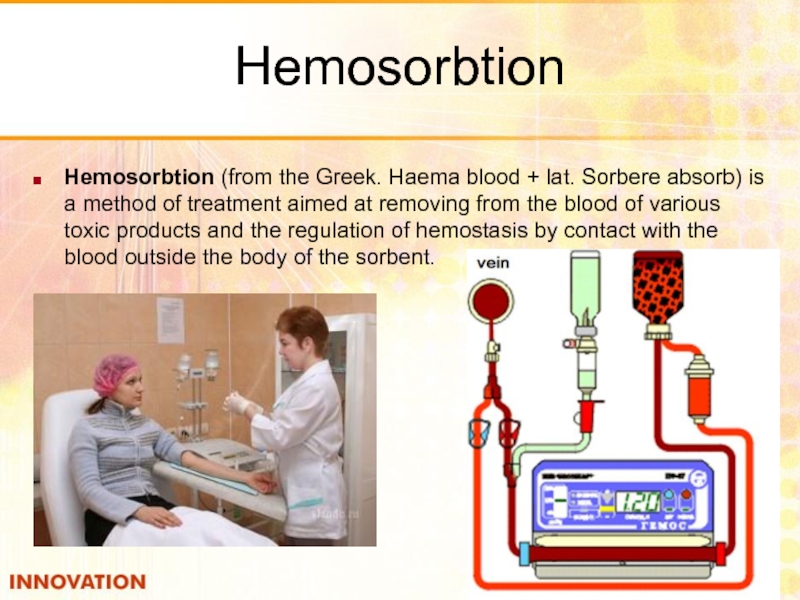
- 53. Hemosorbtion Hemosorbtion (from the Greek. Haema blood
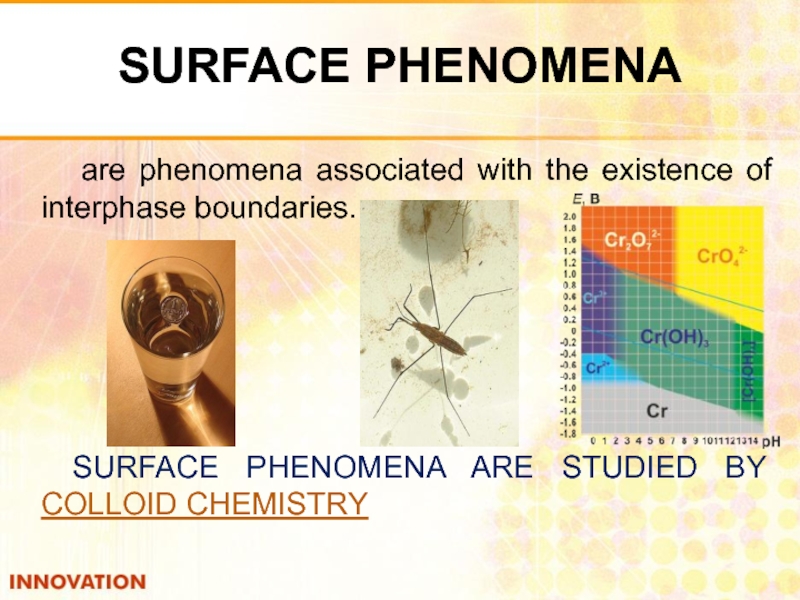
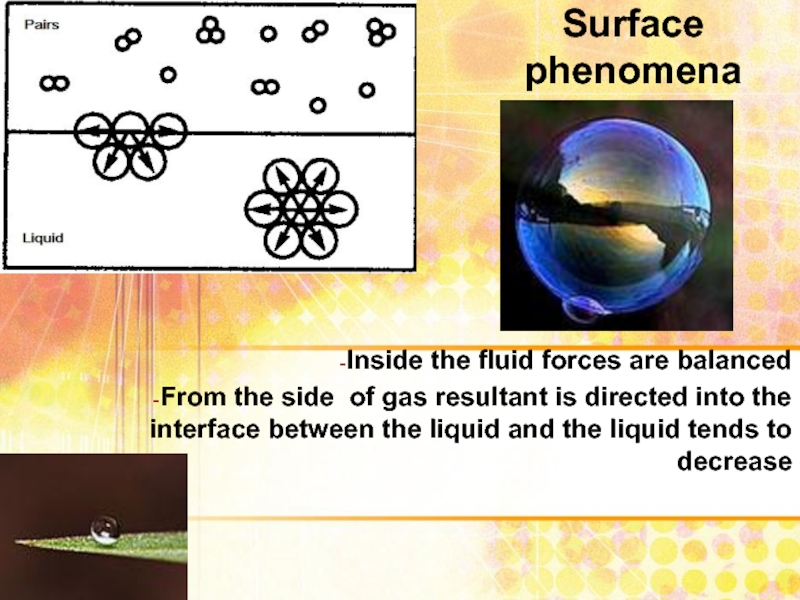
Слайд 2SURFACE PHENOMENA
are phenomena associated with the existence of interphase boundaries.
SURFACE PHENOMENA
Слайд 3Surface phenomena
Inside the fluid forces are balanced
From the side of
Слайд 4Surface phenomena
The increased surface area of the phase separation is associated
-dW=σ·dS
σ- the coefficient of proportionality, called surface tension.
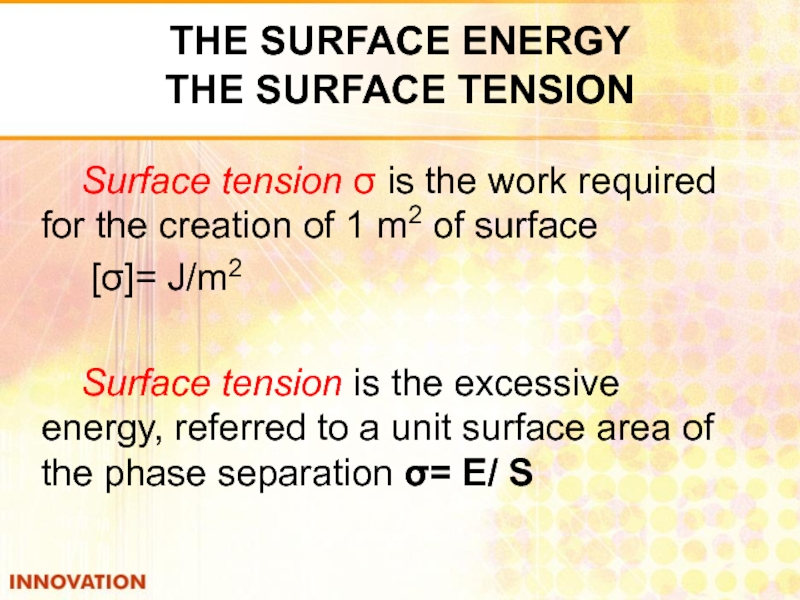
Слайд 5Surface tension σ is the work required for the creation of
[σ]= J/m2
Surface tension is the excessive energy, referred to a unit surface area of the phase separation σ= Е/ S
THE SURFACE ENERGY
THE SURFACE TENSION
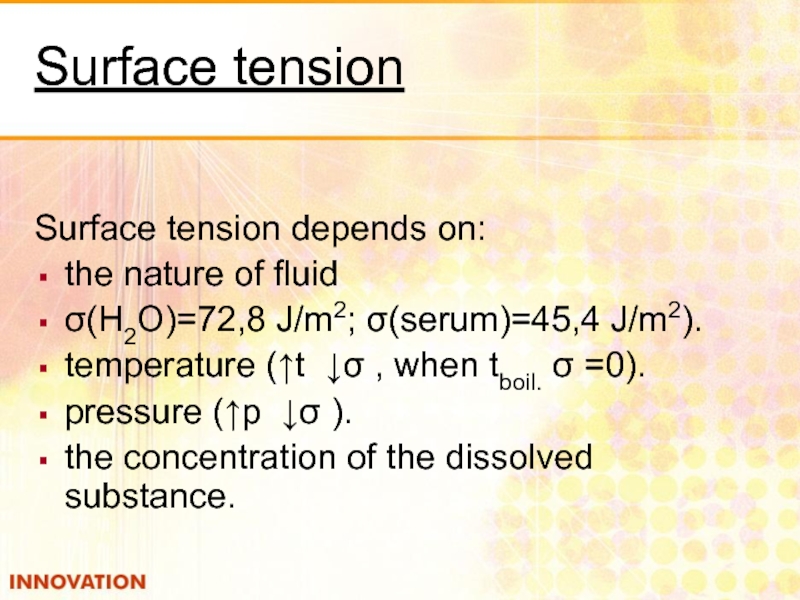
Слайд 6Surface tension
Surface tension depends on:
the nature of fluid
σ(Н2О)=72,8 J/m2; σ(serum)=45,4 J/m2).
temperature
pressure (↑p ↓σ ).
the concentration of the dissolved substance.
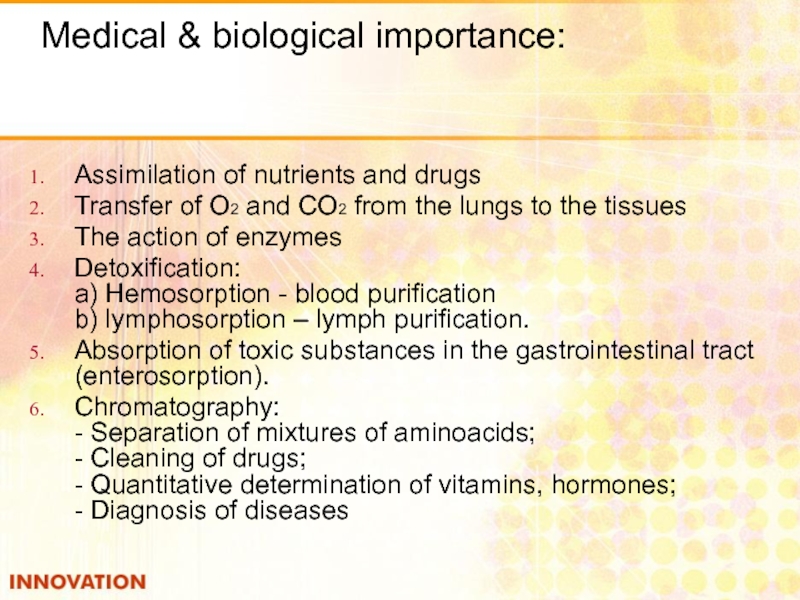
Слайд 8Medical & biological importance:
Assimilation of nutrients and drugs
Transfer of
The action of enzymes
Detoxification: a) Hemosorption - blood purification b) lymphosorption – lymph purification.
Absorption of toxic substances in the gastrointestinal tract (enterosorption).
Chromatography: - Separation of mixtures of aminoacids; - Cleaning of drugs; - Quantitative determination of vitamins, hormones; - Diagnosis of diseases
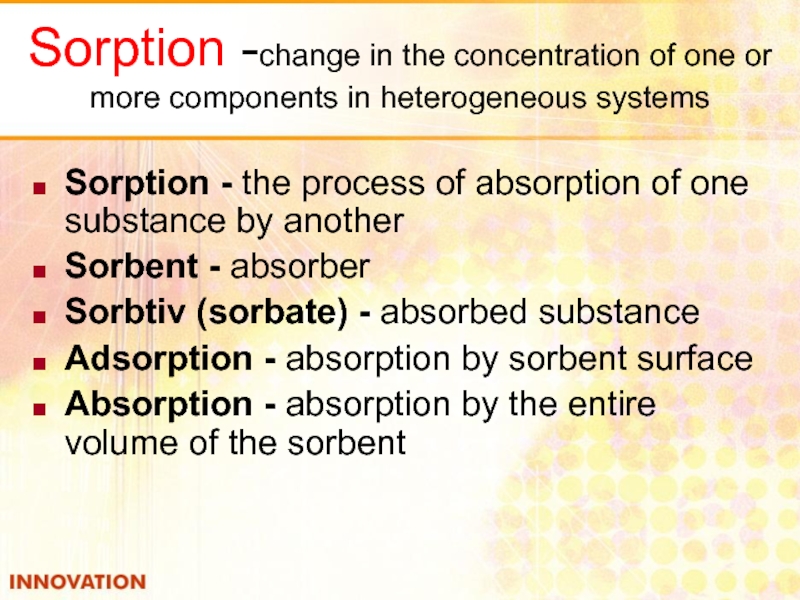
Слайд 9Sorption -change in the concentration of one or more components in
Sorption - the process of absorption of one substance by another
Sorbent - absorber
Sorbtiv (sorbate) - absorbed substance
Adsorption - absorption by sorbent surface
Absorption - absorption by the entire volume of the sorbent
Слайд 10Adsorption
Adsorption is spontaneous change of component concentration in the surface layer
Слайд 11Gibbs Equation
G - the amount of adsorbed substance [mole/m2]
а – equilibrium
G - the amount of adsorbed substance [mole/m2]
с – the concentration of the substance in solution [mole/l]
R - universal gas constant = 8,31 J/моль∙(°К)
G - the amount adsorbed substance [mole/m2]
р – the equilibrium gas pressure, Pa
R - universal gas constant = 8,31 J/моль∙(°К))
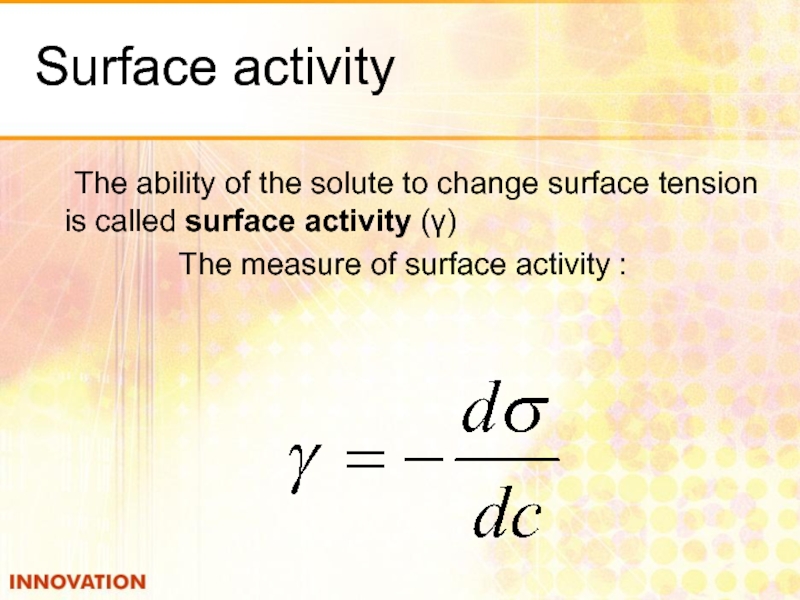
Слайд 12Surface activity
The ability of the solute to change surface tension is
The measure of surface activity :
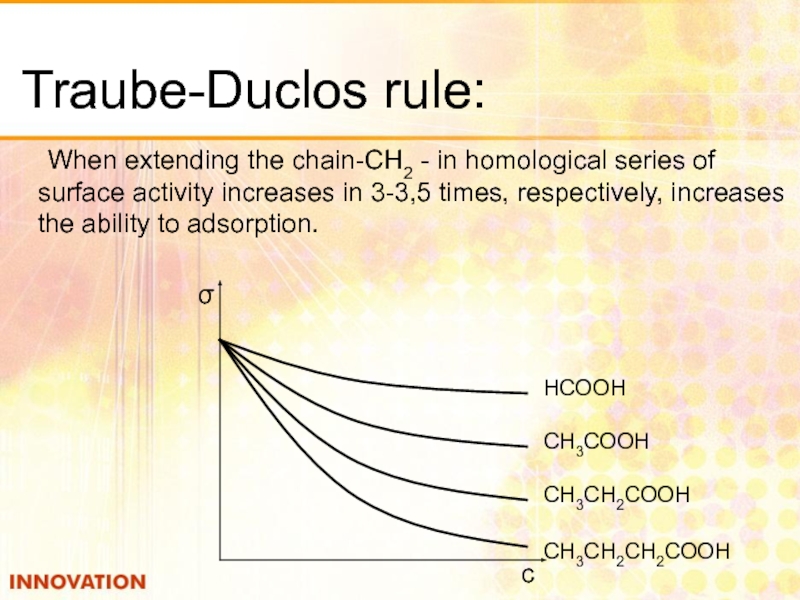
Слайд 13Traube-Duclos rule:
When extending the chain-CH2 - in homological series of surface
НСООН
СН3СООН
СН3СН2СООН
СН3СН2СН2СООН
σ
с
Слайд 14SAS, SIS, SNS
Surface-active substances (SAS):
reduce σ solvent. σ solution
Surface-inactive substance (SIS): increase σ of solvent. σ solution > σ solvent; g
Surfactants-nonactive substance (SNS): do not alter the surface tension of the solvent. σ solution = σ solvent; g = O. SNS: sucrose.
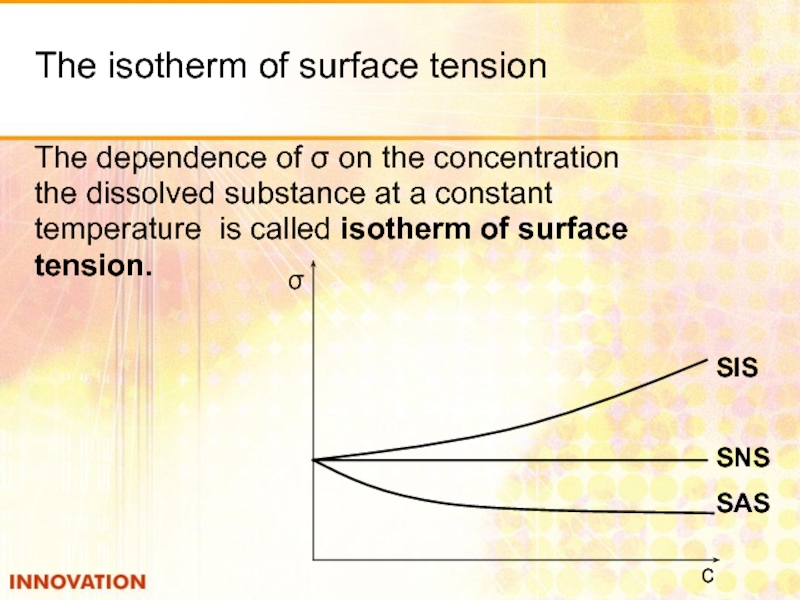
Слайд 15The isotherm of surface tension
The dependence of σ on the concentration
the
temperature is called isotherm of surface
tension.
SIS
SNS
SAS
σ
с
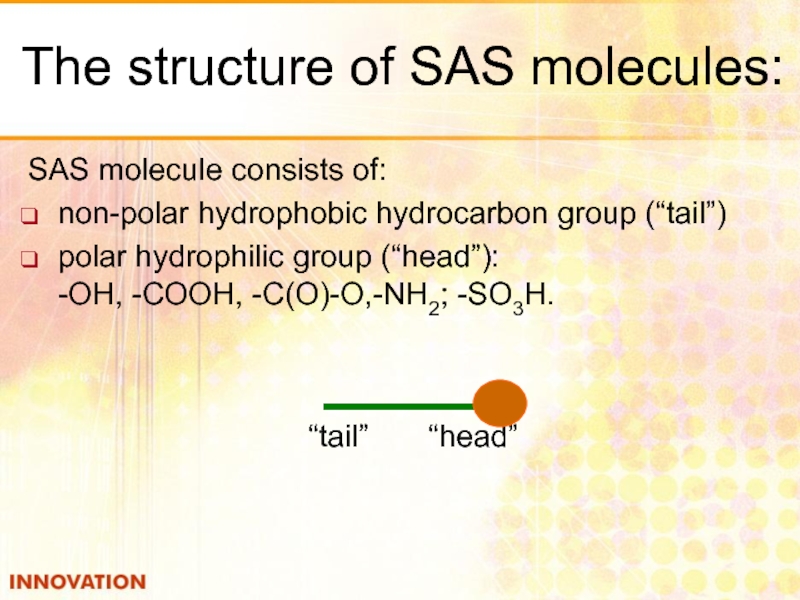
Слайд 16The structure of SAS molecules:
SAS molecule consists of:
non-polar hydrophobic hydrocarbon group
polar hydrophilic group (“head”): -ОН, -СООН, -С(О)-О,-NН2; -SО3H.
“tail” “head”
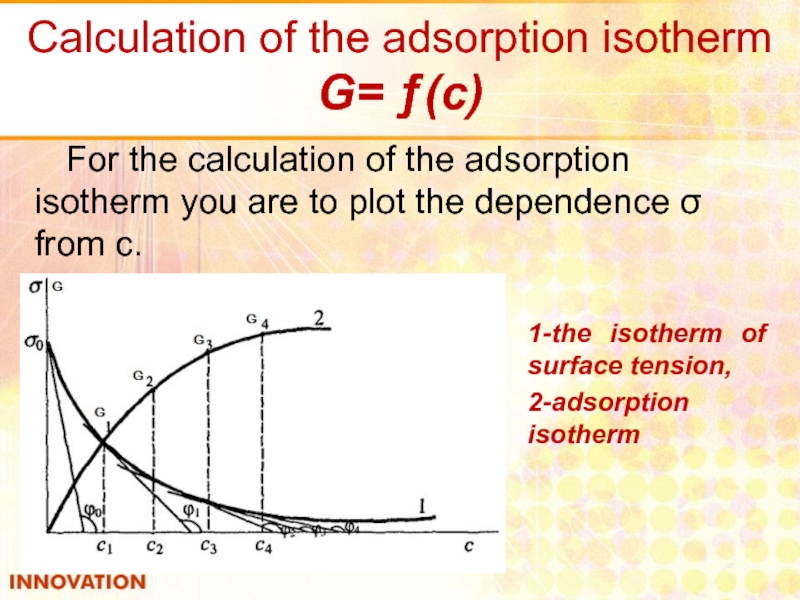
Слайд 18Calculation of the adsorption isotherm
G= ƒ(с)
For the calculation of the
1-the isotherm of surface tension,
2-adsorption isotherm
Слайд 21Adsorption by solids
The adsorption value depends on:
1. The size of the
if S↑ surface then adsorption ↑.
2. Temperature (↑t ↓G ).
3. Type of sorbent affinity thereof to the solvent. - Hydrophilic. - Hydrophobic.
4. Charge of the adsorbent and the adsorptive.
5. Adsorptive concentration.
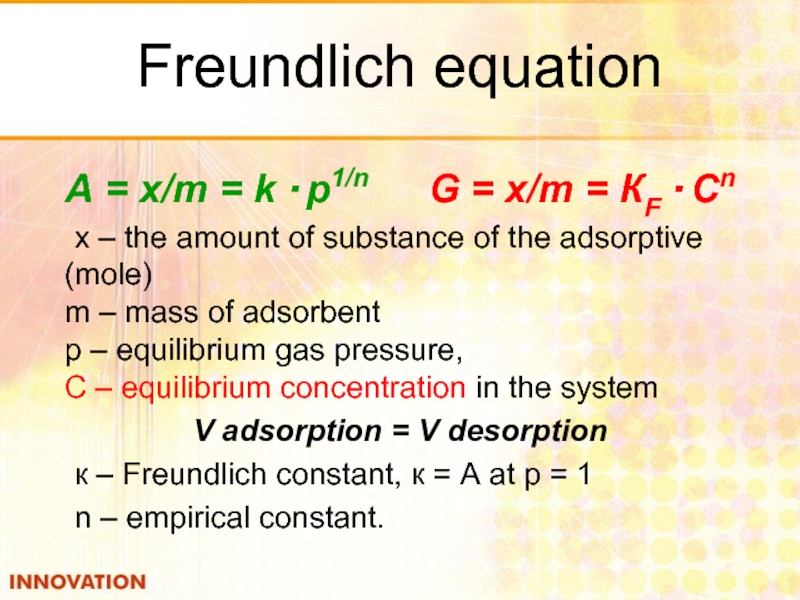
Слайд 22Freundlich equation
А = x/m = k · p1/n
х – the amount of substance of the adsorptive (mole) m – mass of adsorbent p – equilibrium gas pressure, С – equilibrium concentration in the system
V adsorption = V desorption
к – Freundlich constant, к = А at р = 1
n – empirical constant.
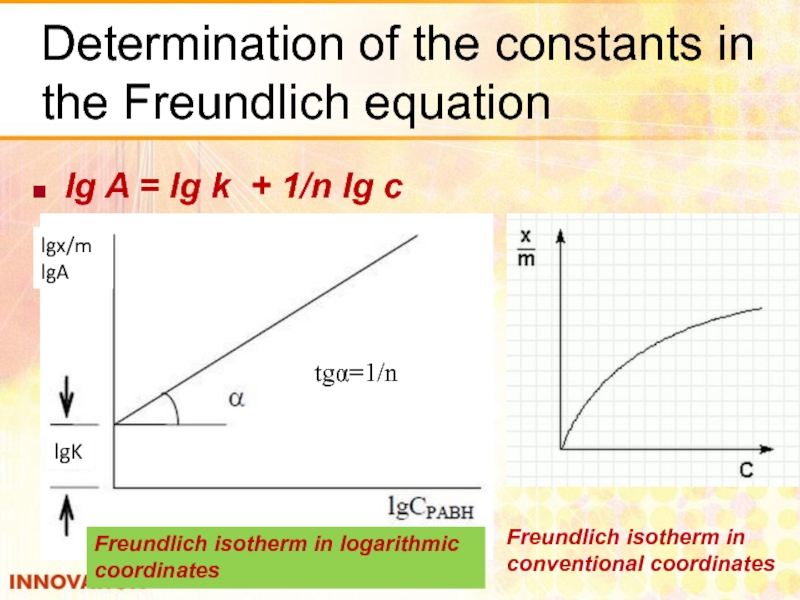
Слайд 23Determination of the constants in the Freundlich equation
lg A = lg
lgx/m
lgA
lgK
tgα=1/n
Freundlich isotherm in
conventional coordinates
Freundlich isotherm in logarithmic coordinates
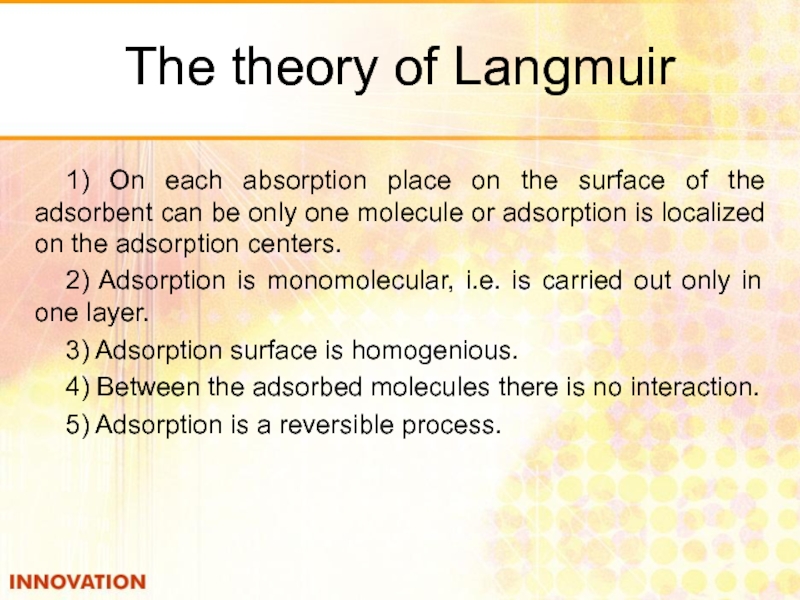
Слайд 24The theory of Langmuir
1) On each absorption place on the surface
2) Adsorption is monomolecular, i.e. is carried out only in one layer.
3) Adsorption surface is homogenious.
4) Between the adsorbed molecules there is no interaction.
5) Adsorption is a reversible process.
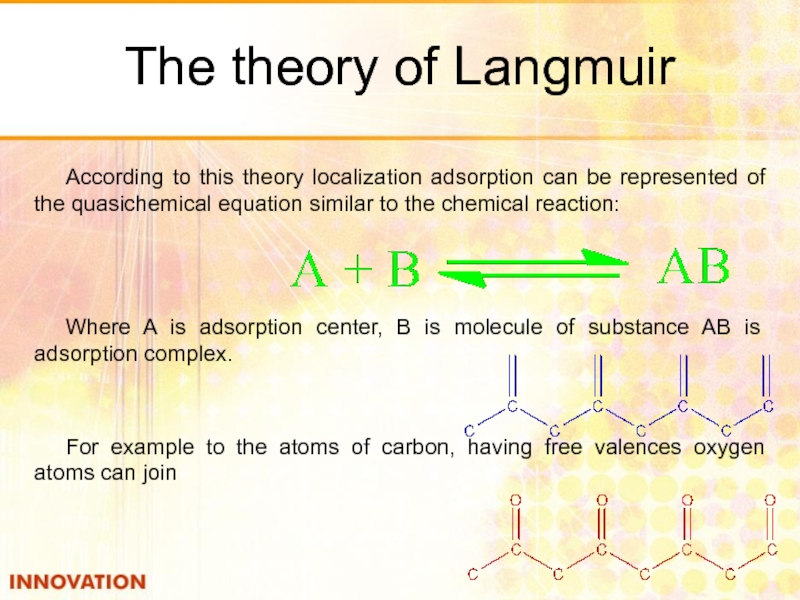
Слайд 25The theory of Langmuir
According to this theory localization adsorption can be
Where A is adsorption center, B is molecule of substance AB is adsorption complex.
For example to the atoms of carbon, having free valences oxygen atoms can join
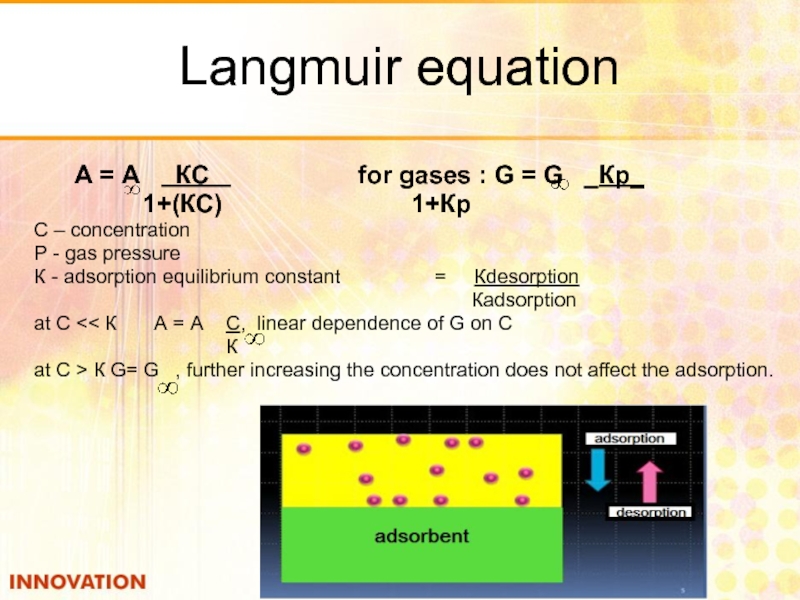
Слайд 26Langmuir equation
А = А КС
1+(КС) 1+Кр
С – concentration
Р - gas pressure
К - adsorption equilibrium constant = Кdesorption
Кadsorption
at С << К А = А С, linear dependence of G on С
К
at С > К G= G , further increasing the concentration does not affect the adsorption.
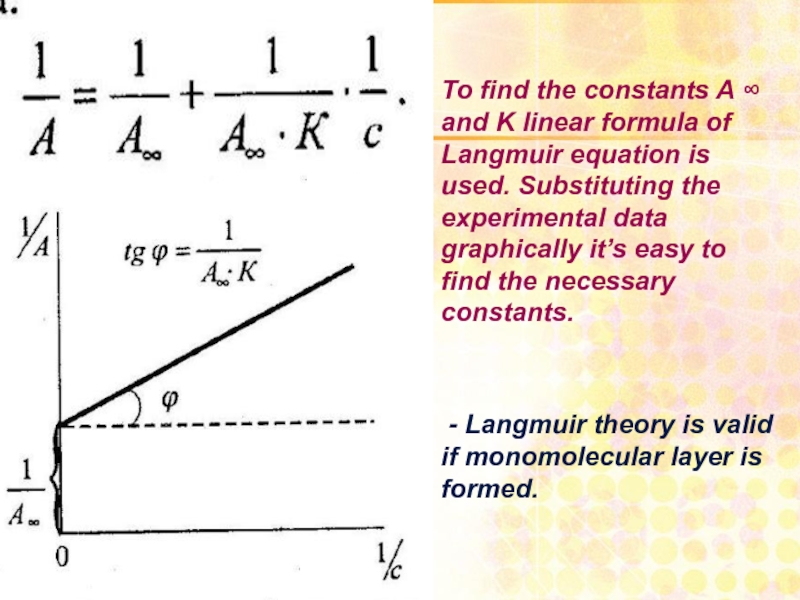
Слайд 27
To find the constants A ∞ and K linear formula of
- Langmuir theory is valid if monomolecular layer is formed.
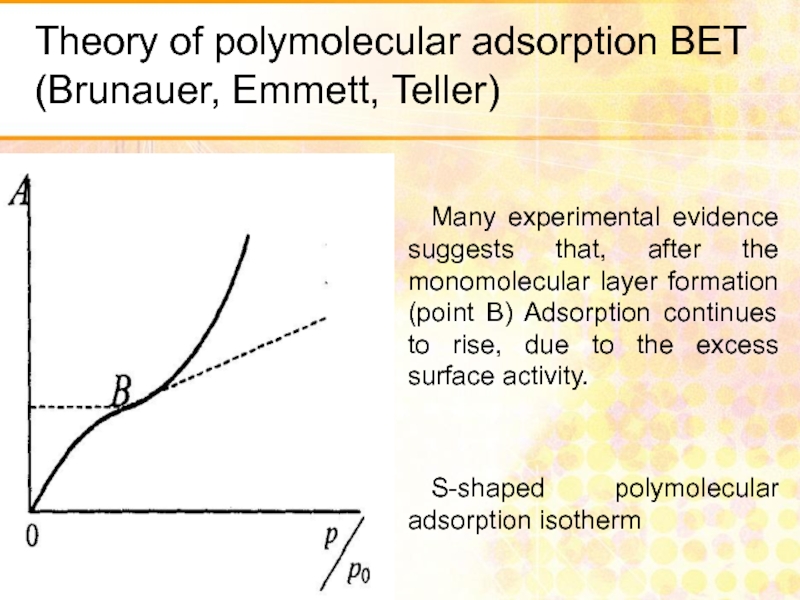
Слайд 29Theory of polymolecular adsorption BET (Brunauer, Emmett, Teller)
Many experimental evidence suggests
S-shaped polymolecular adsorption isotherm
Слайд 30ADSORPTION ON THE BORDER OF
SOLID – SOLUTION
In the study of
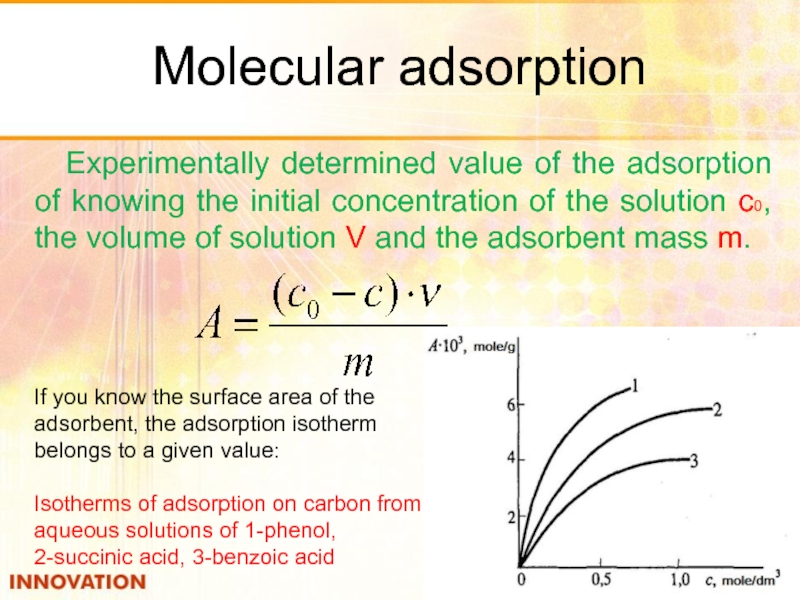
Слайд 31Molecular adsorption
Experimentally determined value of the adsorption of knowing the initial
If you know the surface area of the adsorbent, the adsorption isotherm
belongs to a given value:
Isotherms of adsorption on carbon from aqueous solutions of 1-phenol,
2-succinic acid, 3-benzoic acid
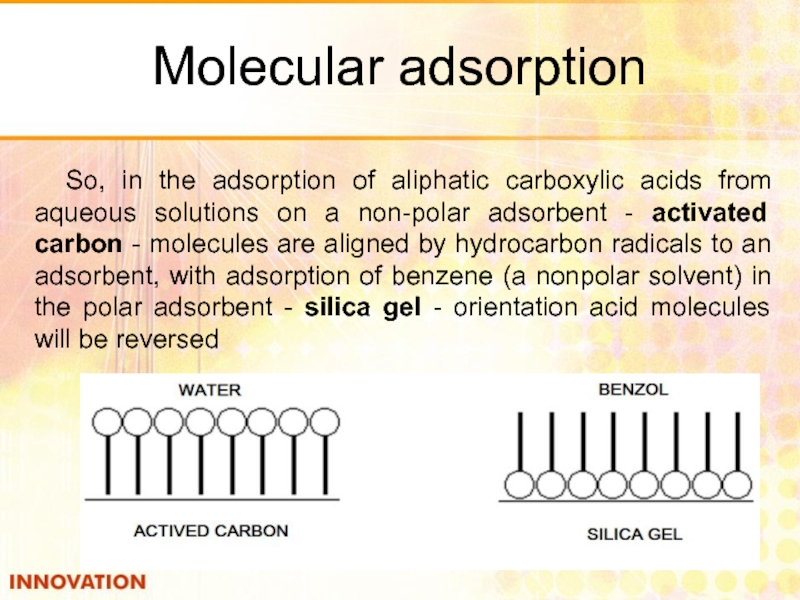
Слайд 32Molecular adsorption
So, in the adsorption of aliphatic carboxylic acids from aqueous
Слайд 33Conclusion
From the above that is confirmed, that:
For adsorption SAS from
On the surfaces of hydrophobic (coal, graphite, talc) from aqueous solutions of SAS should be better adsorbed.
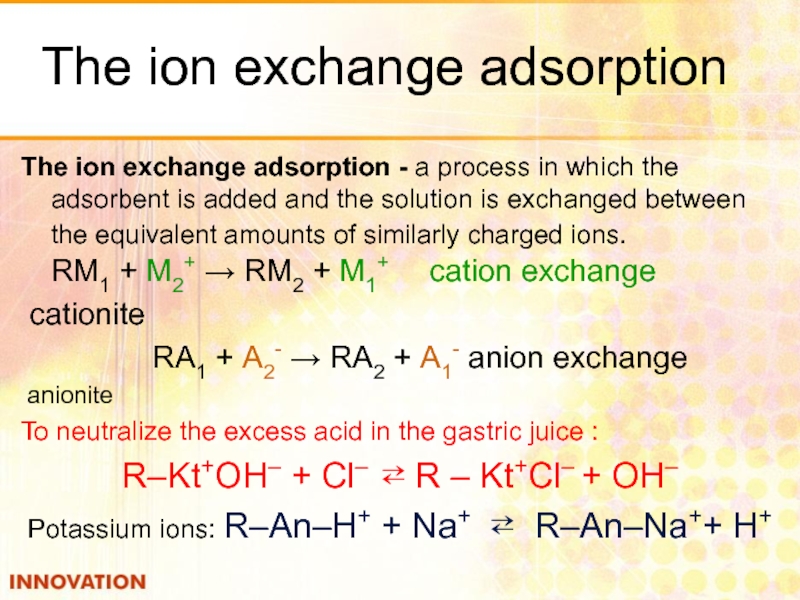
Слайд 34The ion exchange adsorption
The ion exchange adsorption - a process in
cationite
RА1 + А2- → RА2 + А1- anion exchange
anionite
To neutralize the excess acid in the gastric juice :
R–Kt+OH– + Cl– ⇄ R – Kt+Cl– + OH–
Potassium ions: R–An–H+ + Na+ ⇄ R–An–Na++ H+
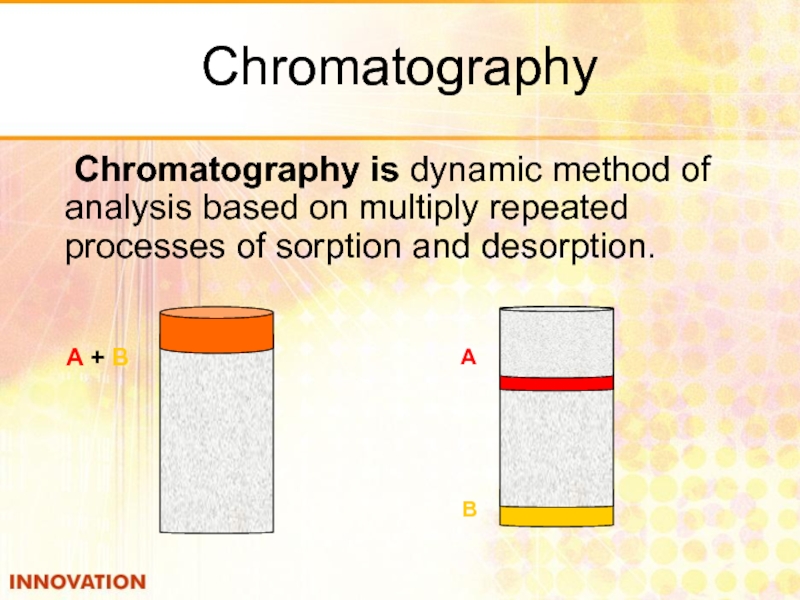
Слайд 35Chromatography
Chromatography is dynamic method of analysis based on multiply repeated processes
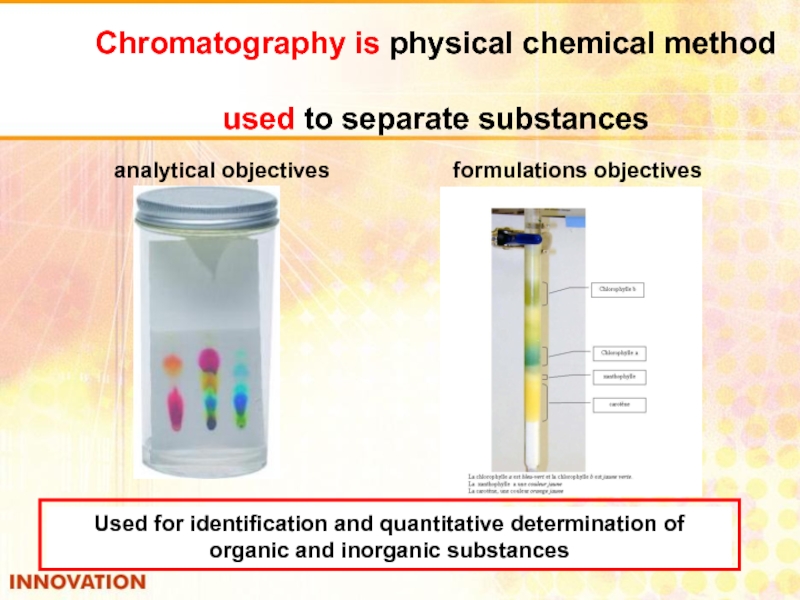
Слайд 36Chromatography is physical chemical method
used to separate substances
analytical objectives
Used for identification and quantitative determination of organic and inorganic substances
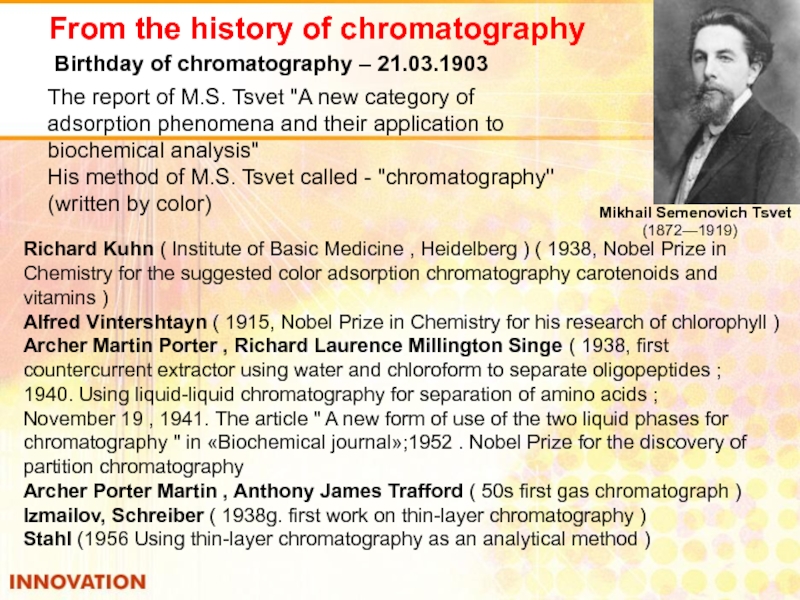
Слайд 37From the history of chromatography
Mikhail Semenovich Tsvet
Birthday of chromatography – 21.03.1903
The report of M.S. Tsvet "A new category of adsorption phenomena and their application to biochemical analysis"
His method of M.S. Tsvet called - "chromatography" (written by color)
Richard Kuhn ( Institute of Basic Medicine , Heidelberg ) ( 1938, Nobel Prize in Chemistry for the suggested color adsorption chromatography carotenoids and vitamins )
Alfred Vintershtayn ( 1915, Nobel Prize in Chemistry for his research of chlorophyll )
Archer Martin Porter , Richard Laurence Millington Singe ( 1938, first countercurrent extractor using water and chloroform to separate oligopeptides ;
1940. Using liquid-liquid chromatography for separation of amino acids ;
November 19 , 1941. The article " A new form of use of the two liquid phases for chromatography " in «Biochemical journal»;1952 . Nobel Prize for the discovery of partition chromatography
Archer Porter Martin , Anthony James Trafford ( 50s first gas chromatograph )
Izmailov, Schreiber ( 1938g. first work on thin-layer chromatography )
Stahl (1956 Using thin-layer chromatography as an analytical method )
Слайд 38«No other discovery had such a huge long lasting effect in
Carrere, 1947.
Chromatographic methods are used for:
quantitative assessment of the basic substance in the bulk drug;
determination of impurities in bulk drug and medicinal forms;
the preliminary and confirming stages in the pharmaceutical, chemical and toxicological analysis;
determining the purity of water and food;
studying the kinetics of chemical reactions;
analyzing oil, etc.
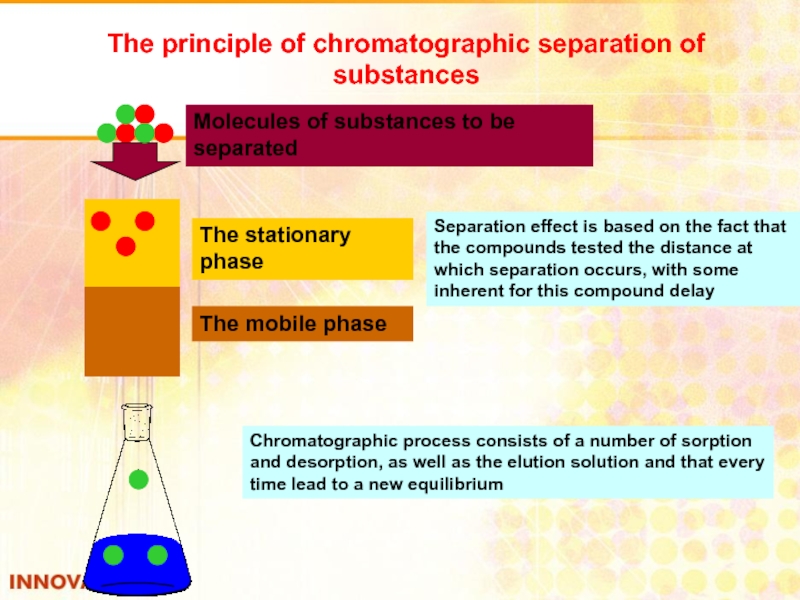
Слайд 39The principle of chromatographic separation of substances
The stationary phase
The mobile phase
Molecules
Separation effect is based on the fact that the compounds tested the distance at which separation occurs, with some inherent for this compound delay
Chromatographic process consists of a number of sorption and desorption, as well as the elution solution and that every time lead to a new equilibrium
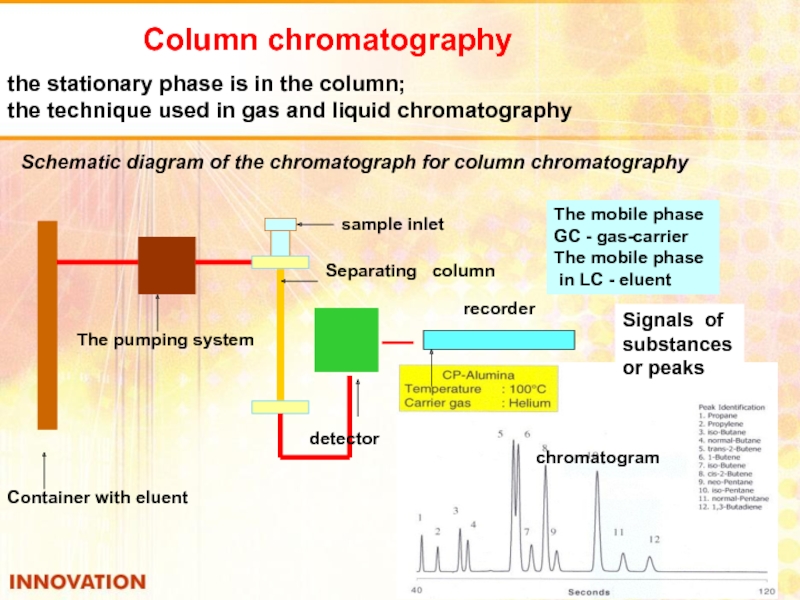
Слайд 40Column chromatography
the stationary phase is in the column;
the technique used in
Schematic diagram of the chromatograph for column chromatography
Container with eluent
The pumping system
sample inlet
Separating column
detector
The mobile phase
GC - gas-carrier
The mobile phase
in LC - eluent
recorder
chromatogram
Signals of substances or peaks




![Gibbs EquationG - the amount of adsorbed substance [mole/m2]а – equilibrium activity of the substances](/img/tmb/5/447648/23f0530ef24a0229d2eb87c3cb3263eb-800x.jpg)