- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Electrochemistry. Oxidation-reduction equilibrium in water solutions презентация
Содержание
- 1. Electrochemistry. Oxidation-reduction equilibrium in water solutions
- 3. Fe + CuCl2 = Cu + FeCl2
- 4. Criteria for spontaneous red-ox reactions Mn+7
- 5. Red-ox potentials of biological systems
- 6. Electrodes
- 7. 1 type-electrodes Me Me+n –
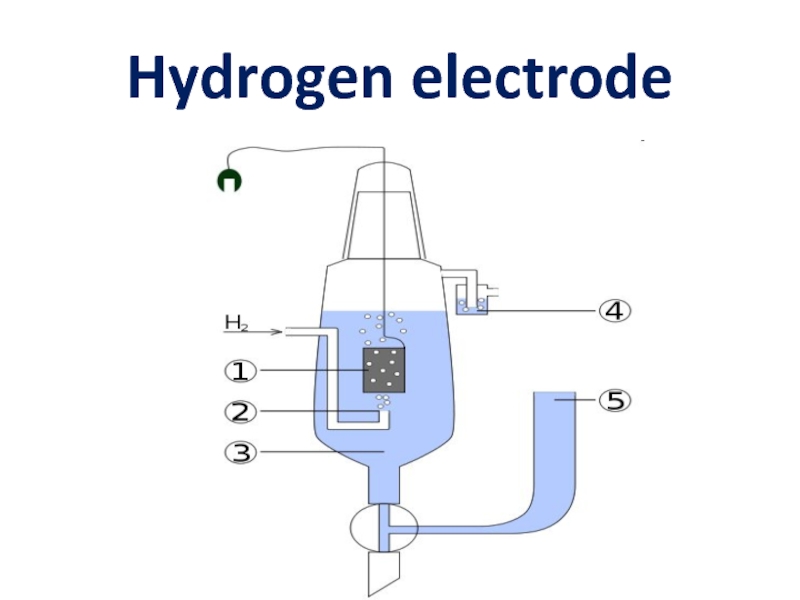
- 8. Hydrogen electrode
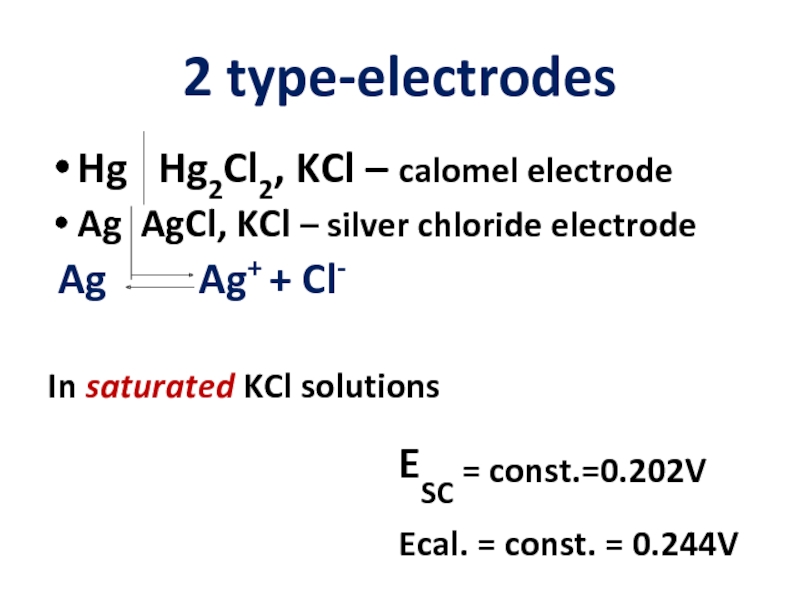
- 9. 2 type-electrodes Hg Hg2Cl2, KCl
- 10. Silver chloride and calomel electrodes
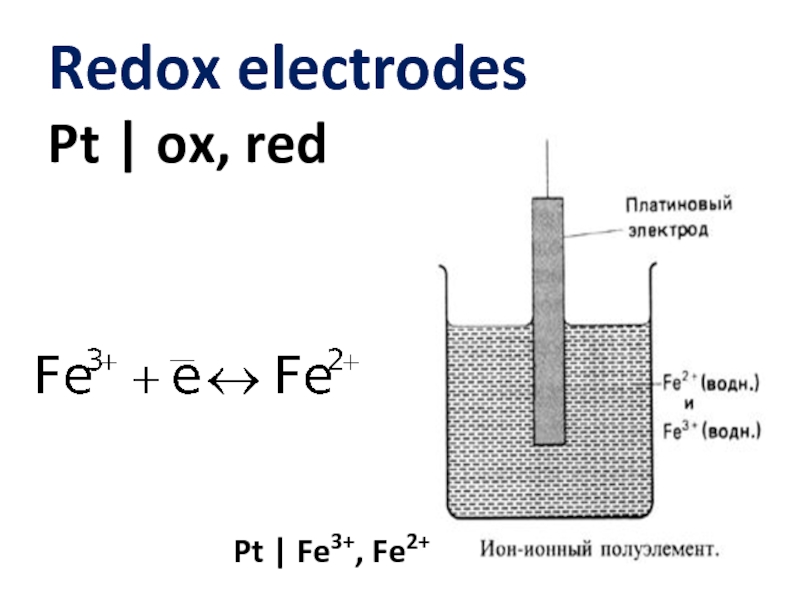
- 11. Redox electrodes Pt | ox, red Pt | Fe3+, Fe2+
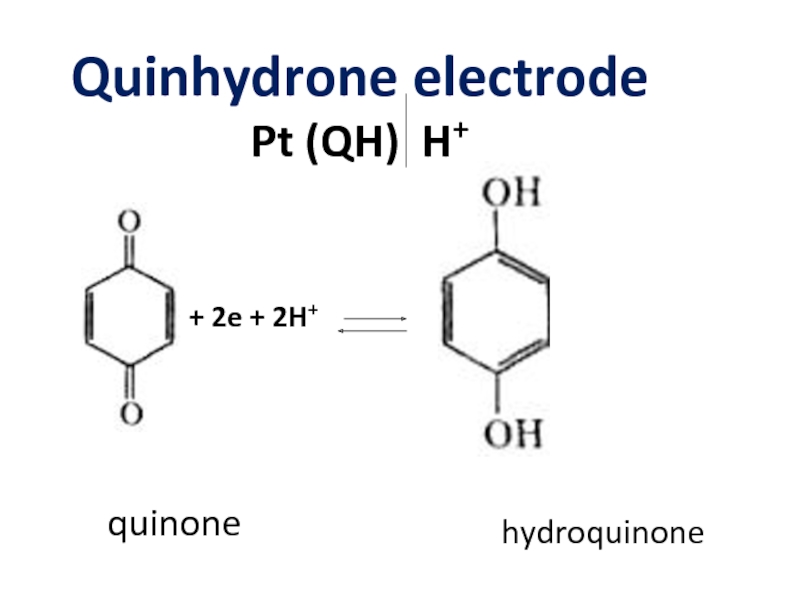
- 12. Quinhydrone electrode Pt (QH) H+ + 2e + 2H+ quinone hydroquinone
- 13. Nernst equation for electrode potential ΔGo
- 14. Hydrogen electrode Pt (H2) | H+
- 15. Nernst equation for biological redox system
- 16. Nernst equation for biological redox system at
- 17. Quinhydrone electrode Pt (QH) H+ Eox/red
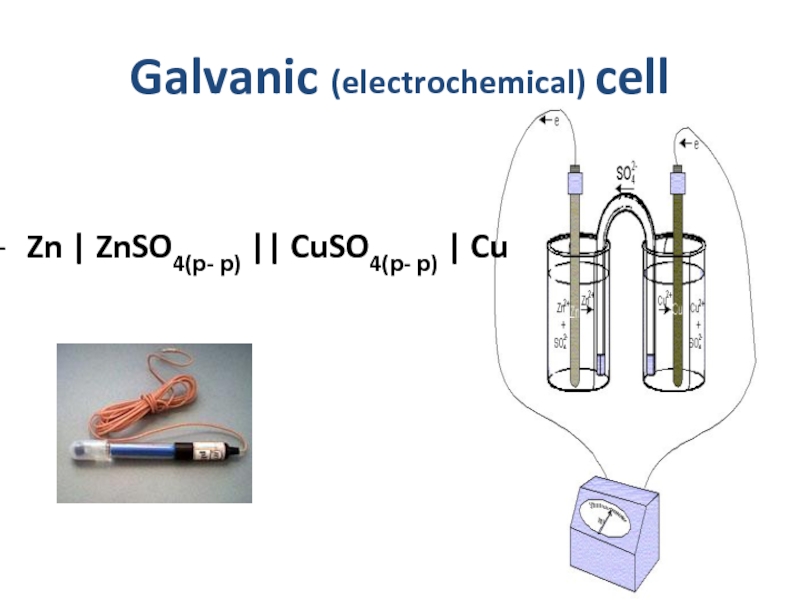
- 18. Galvanic (electrochemical) cell Zn
- 19. electromotive force (EMF)
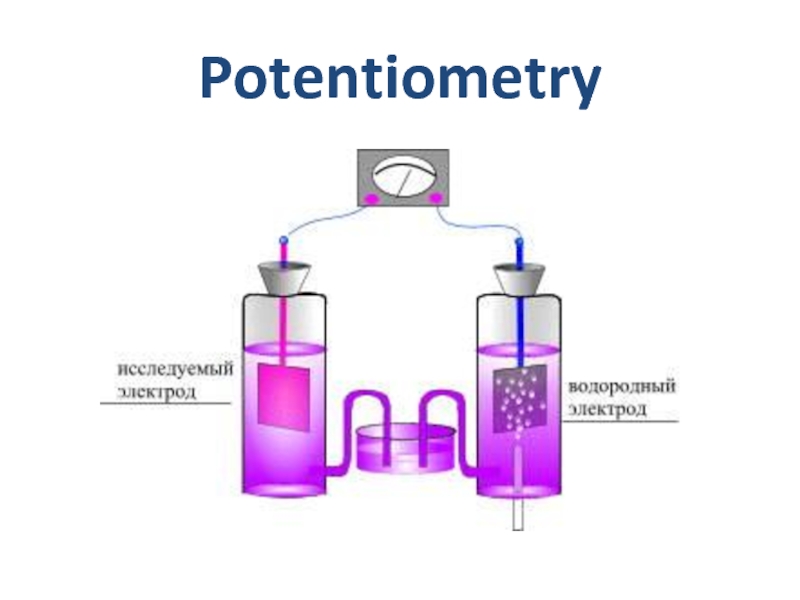
- 20. Potentiometry
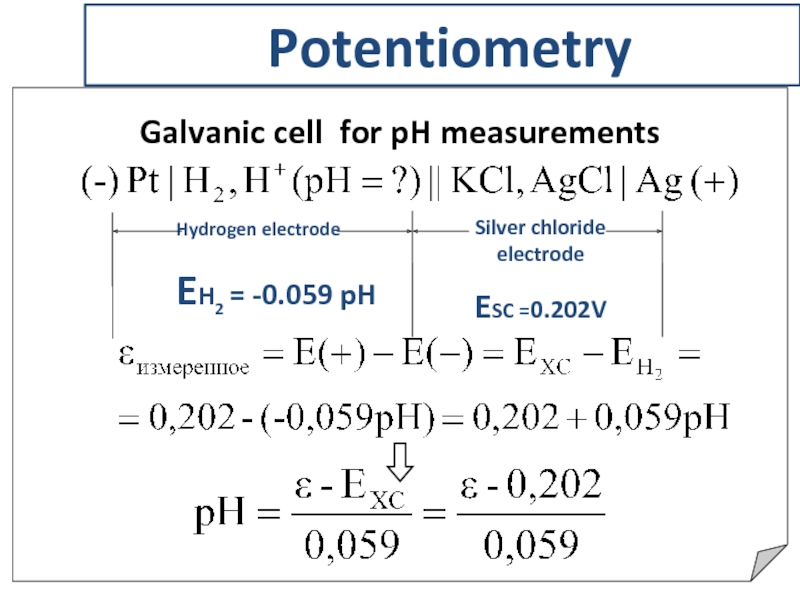
- 21. Potentiometry Galvanic cell for pH measurements
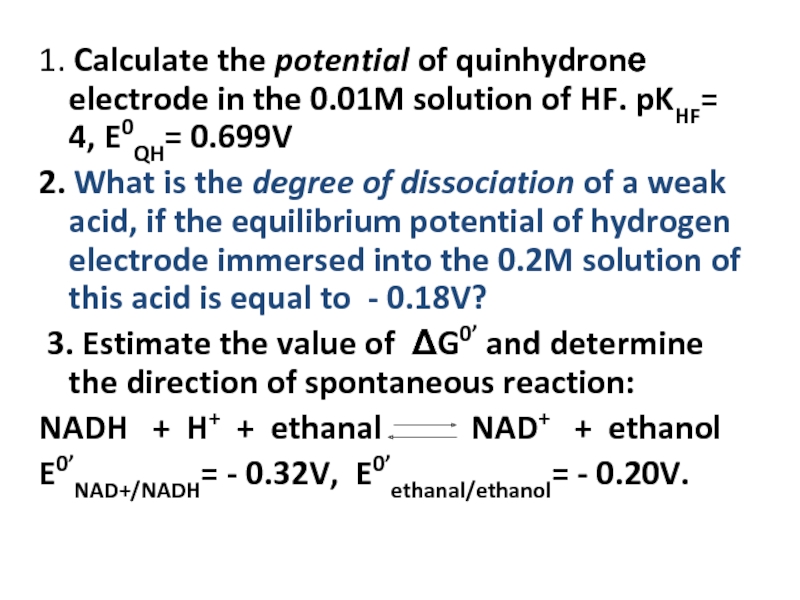
- 22. 1. Calculate the potential of quinhydronе electrode
- 23. 4. Estimate the value of the equilibrium
Слайд 2
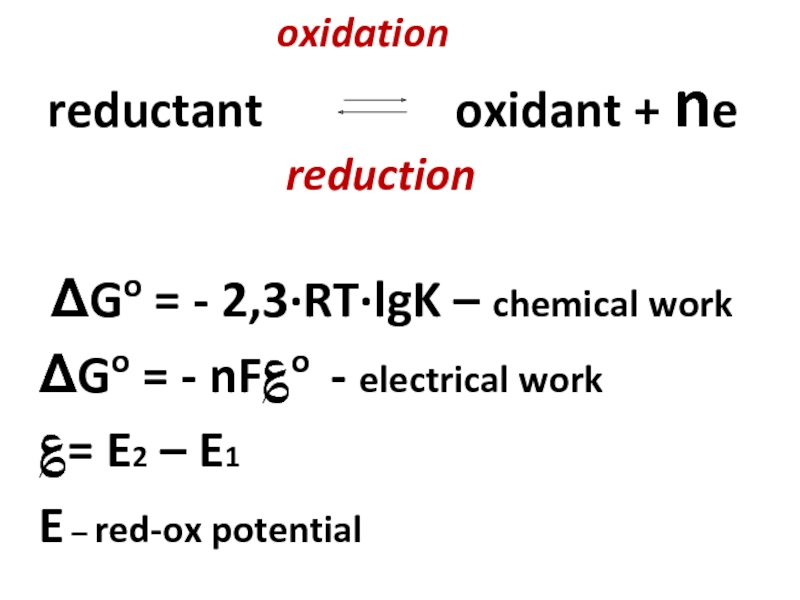
ΔGo = - 2,3·RT·lgK – chemical work
ΔGo = - nF؏o - electrical work
؏= E2 – E1
E – red-ox potential
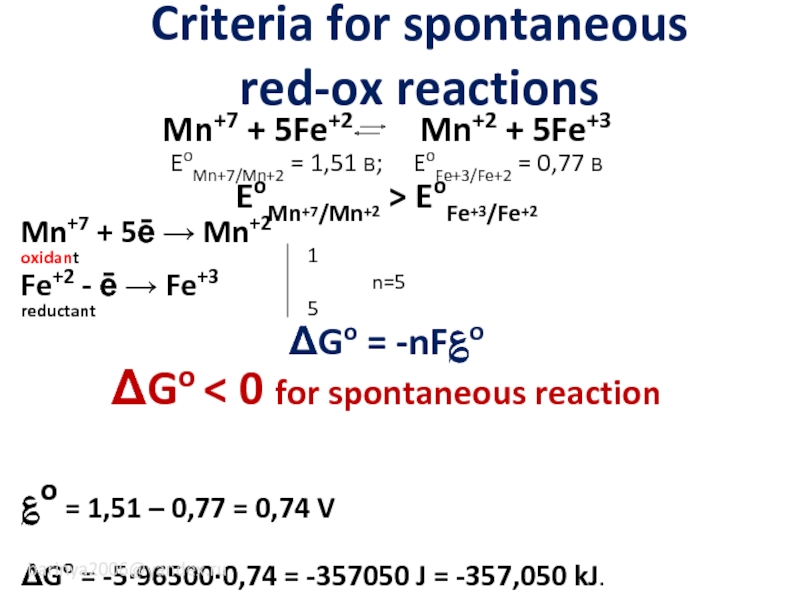
Слайд 4Criteria for spontaneous
red-ox reactions
Mn+7 + 5Fe+2 Mn+2
EoMn+7/Mn+2 = 1,51 В; EoFe+3/Fe+2 = 0,77 В
EoMn+7/Mn+2 > EoFe+3/Fe+2
Mn+7 + 5ē → Mn+2
oxidant
Fe+2 - ē → Fe+3
reductant
ΔGo = -nF؏o
ΔGo < 0 for spontaneous reaction
؏o = 1,51 – 0,77 = 0,74 V
ΔGo = -5·96500·0,74 = -357050 J = -357,050 kJ.
1
n=5
5
barinya2006@yandex.ru
Слайд 5Red-ox potentials
of biological systems
reductant
ΔGo = -2F؏o
Oxidant - acceptor of electrons and protons
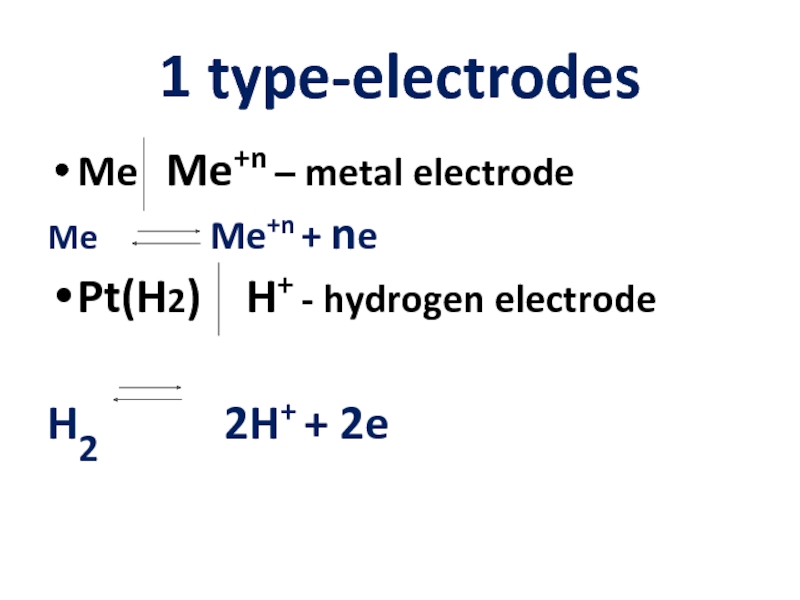
Слайд 71 type-electrodes
Me Me+n – metal electrode
Me
Pt(H2) H+ - hydrogen electrode
H2 2H+ + 2e
Слайд 92 type-electrodes
Hg Hg2Cl2, KCl – calomel electrode
Ag AgCl, KCl
Ag Ag+ + Cl-
In saturated KCl solutions
ESC = const.=0.202V
Ecal. = const. = 0.244V
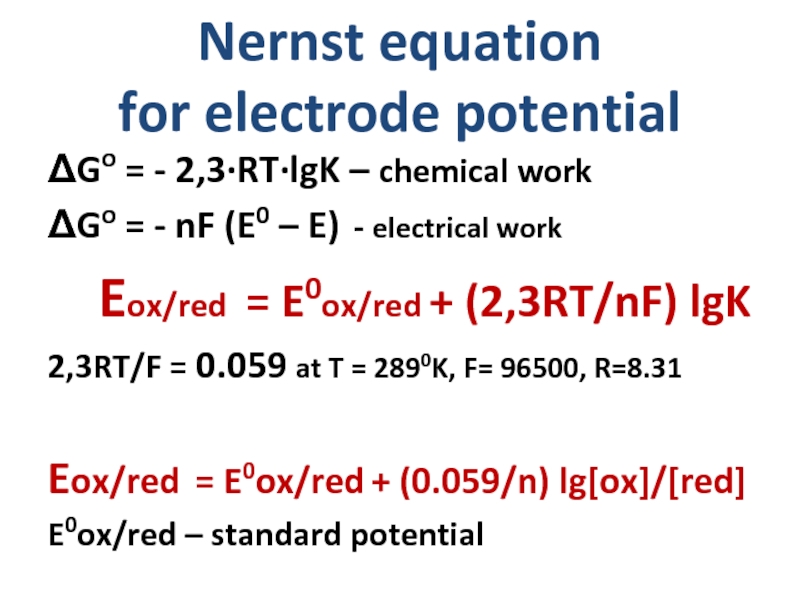
Слайд 13Nernst equation
for electrode potential
ΔGo = - 2,3·RT·lgK – chemical work
ΔGo
Eox/red = E0ox/red + (2,3RT/nF) lgK
2,3RT/F = 0.059 at T = 2890K, F= 96500, R=8.31
Eox/red = E0ox/red + (0.059/n) lg[ox]/[red]
E0ox/red – standard potential
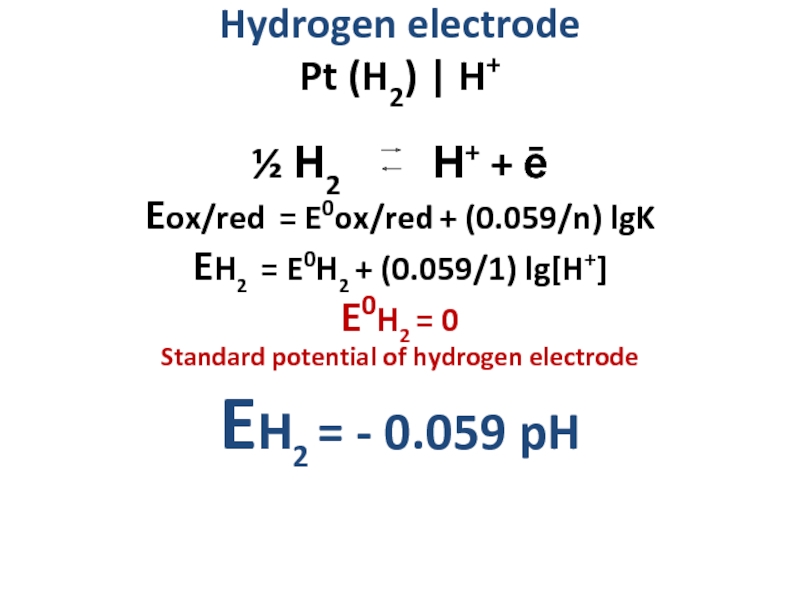
Слайд 14Hydrogen electrode
Pt (H2) | H+
½ Н2 Н+
Eox/red = E0ox/red + (0.059/n) lgK
EН2 = E0Н2 + (0.059/1) lg[H+]
E0Н2 = 0
Standard potential of hydrogen electrode
EН2 = - 0.059 pH
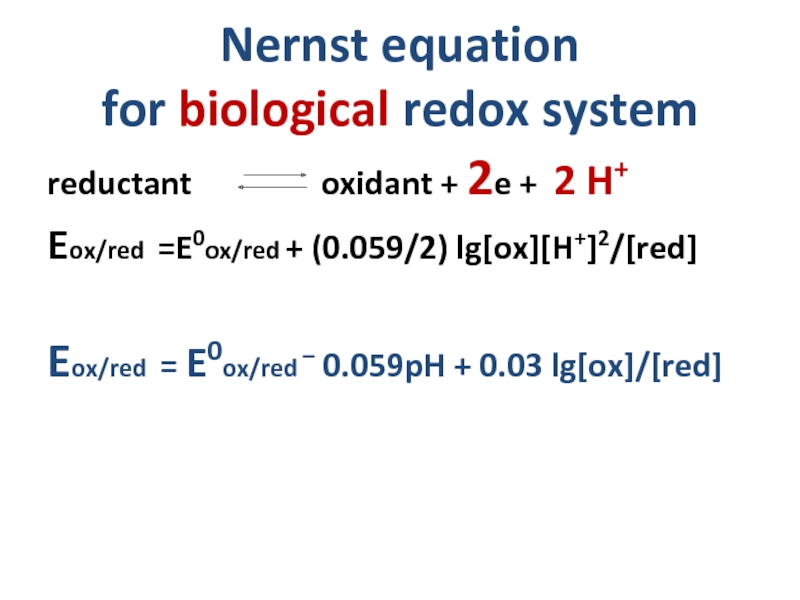
Слайд 15Nernst equation
for biological redox system
reductant
Eox/red =E0ox/red + (0.059/2) lg[ox][H+]2/[red]
Eox/red = E0ox/red – 0.059pH + 0.03 lg[ox]/[red]
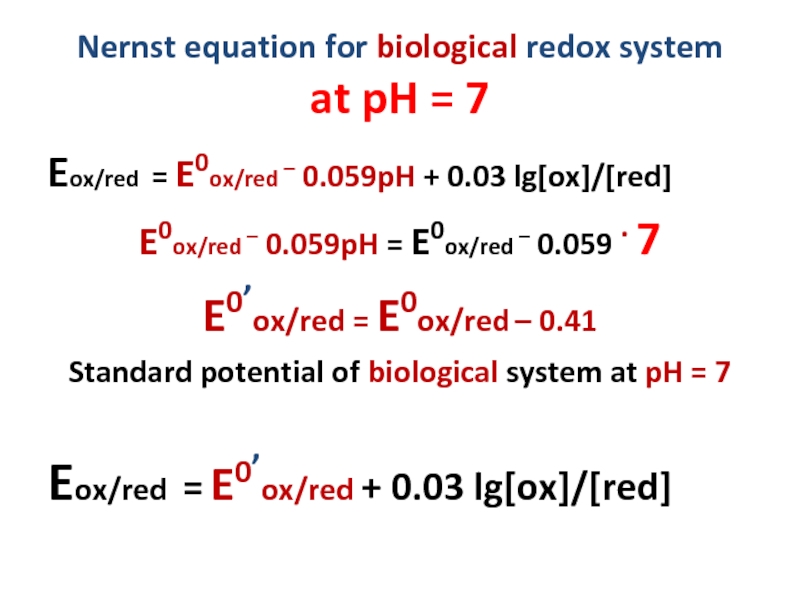
Слайд 16Nernst equation for biological redox system
at pH = 7
Eox/red = E0ox/red
E0ox/red – 0.059pH = E0ox/red – 0.059 . 7
E0’ox/red = E0ox/red – 0.41
Standard potential of biological system at pH = 7
Eox/red = E0’ox/red + 0.03 lg[ox]/[red]
Слайд 17Quinhydrone electrode
Pt (QH) H+
Eox/red = E0ox/red – 0.059pH + 0.03
EQH = E0QH – 0.059pH + 0.03lg[quinone]/[hydroquinone]
Quinhydrone = 1M quinone + 1Mhydroquinone
EQH = E0QH – 0.059pH
Слайд 19 electromotive force (EMF)
؏= E2 –
؏о = ЕoCu – ЕoZn = 0,34 – ( - 0,76) = 1,1B
CuSO4 + Zn → ZnSO4 + Cu
reductant Zno - 2ē → Zn2+ oxidation
oxidant Cu2+ + 2ē → Cuo reduction
barinya2006@yandex.ru
Слайд 21Potentiometry
Galvanic cell for pH measurements
Silver chloride electrode
ESC =0.202V
Hydrogen electrode
EH2 =
Слайд 221. Calculate the potential of quinhydronе electrode in the 0.01M solution
2. What is the degree of dissociation of a weak acid, if the equilibrium potential of hydrogen electrode immersed into the 0.2M solution of this acid is equal to - 0.18V?
3. Estimate the value of ΔG0’ and determine the direction of spontaneous reaction:
NADH + H+ + ethanal NAD+ + ethanol
E0’NAD+/NADH= - 0.32V, E0’ethanal/ethanol= - 0.20V.
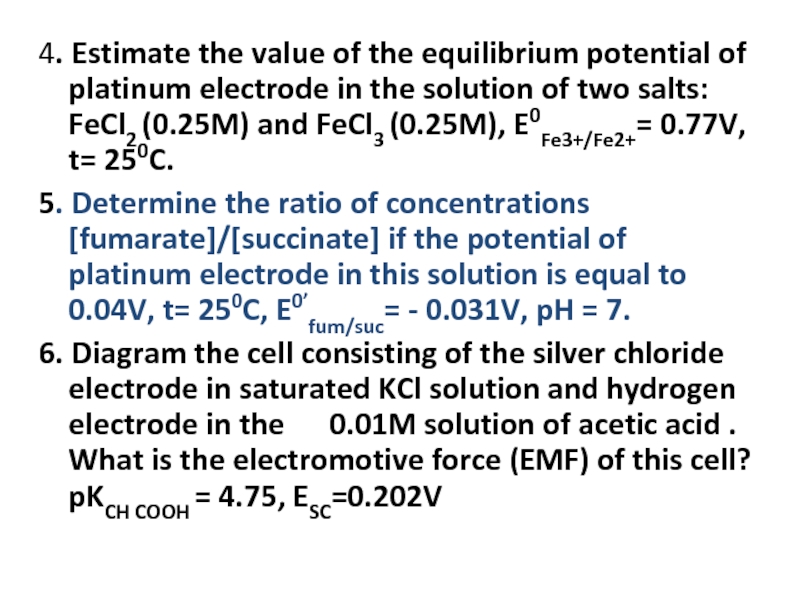
Слайд 234. Estimate the value of the equilibrium potential of platinum electrode
5. Determine the ratio of concentrations [fumarate]/[succinate] if the potential of platinum electrode in this solution is equal to 0.04V, t= 250C, E0’fum/suc= - 0.031V, pH = 7.
6. Diagram the cell consisting of the silver chloride electrode in saturated KCl solution and hydrogen electrode in the 0.01M solution of acetic acid . What is the electromotive force (EMF) of this cell? pKCH COOH = 4.75, ESC=0.202V





![Quinhydrone electrode Pt (QH) H+Eox/red = E0ox/red – 0.059pH + 0.03 lg[ox]/[red] EQH = E0QH](/img/tmb/4/330299/23dd1d5073eba32a222fb82a0f499078-800x.jpg)