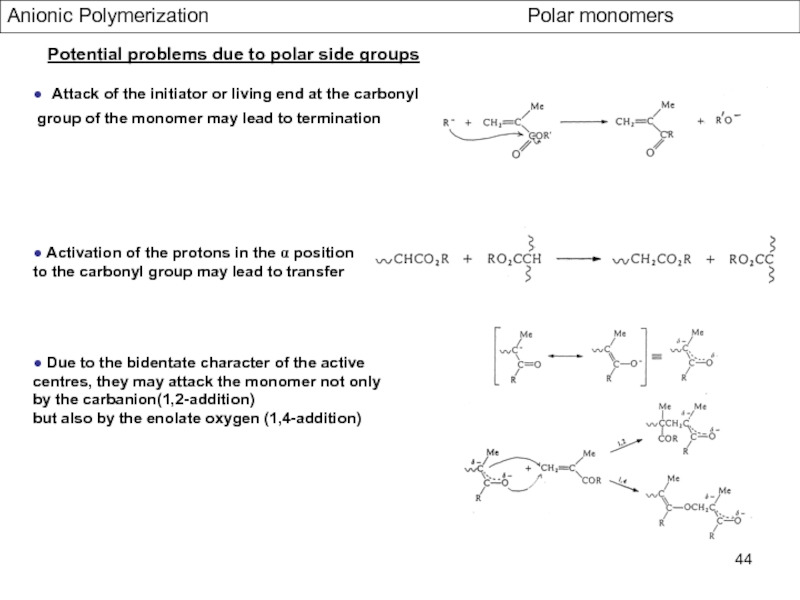
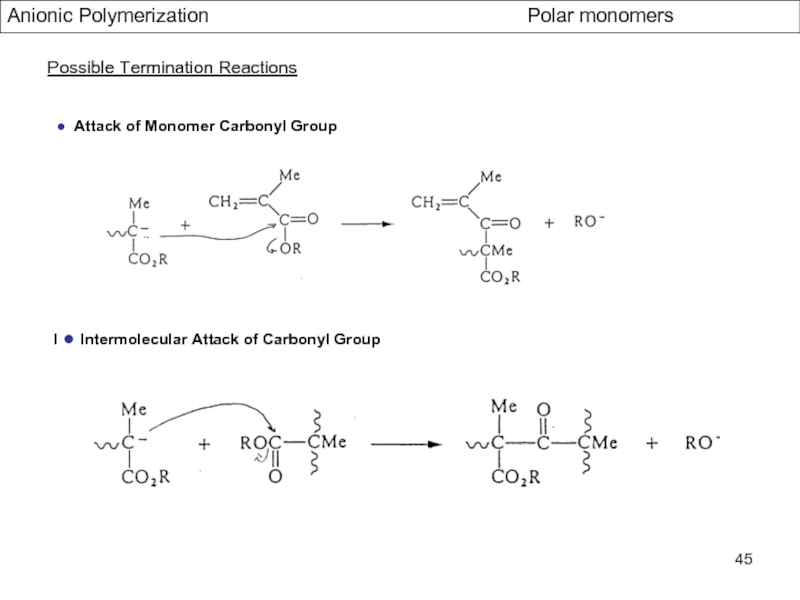
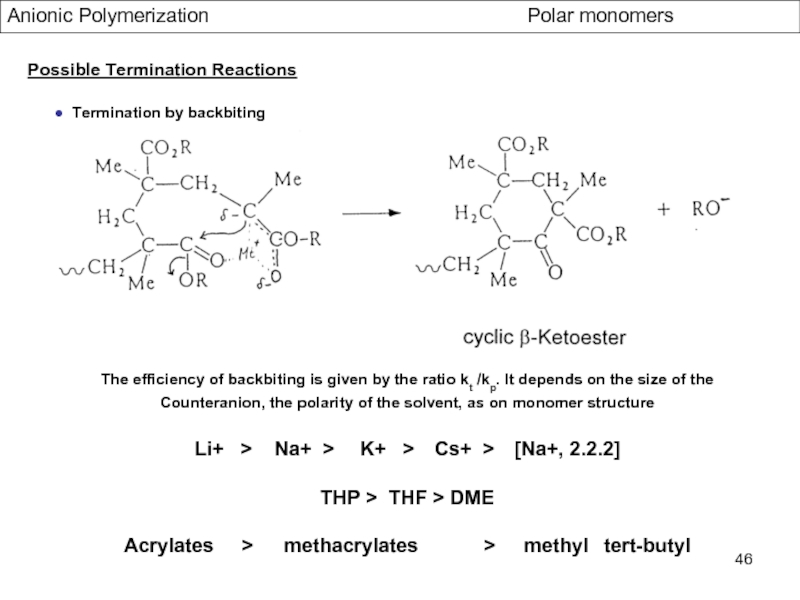
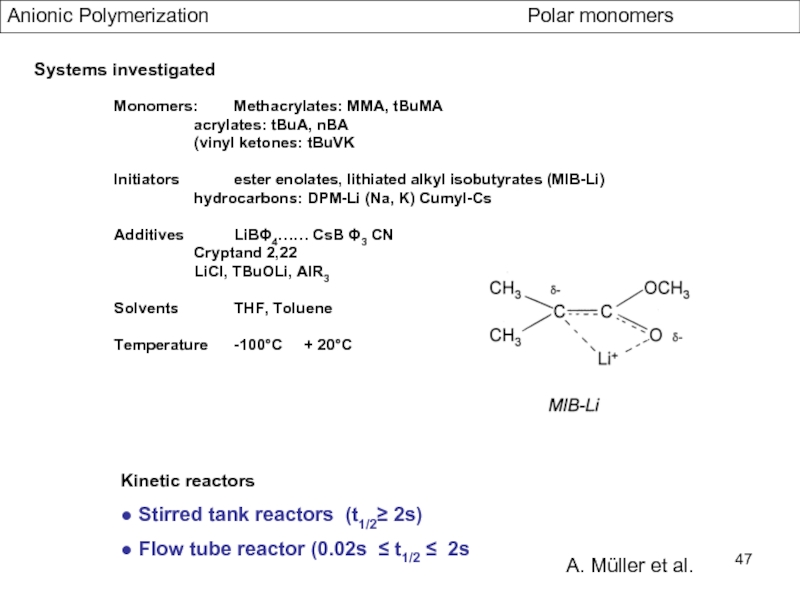
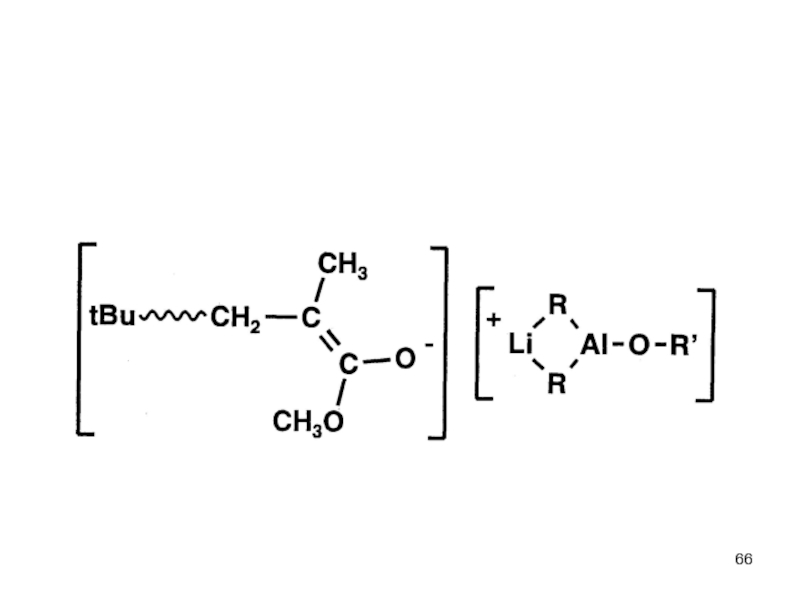
- Aspects of living Polymerization
- Factors Affecting the Molar Mass Distribution
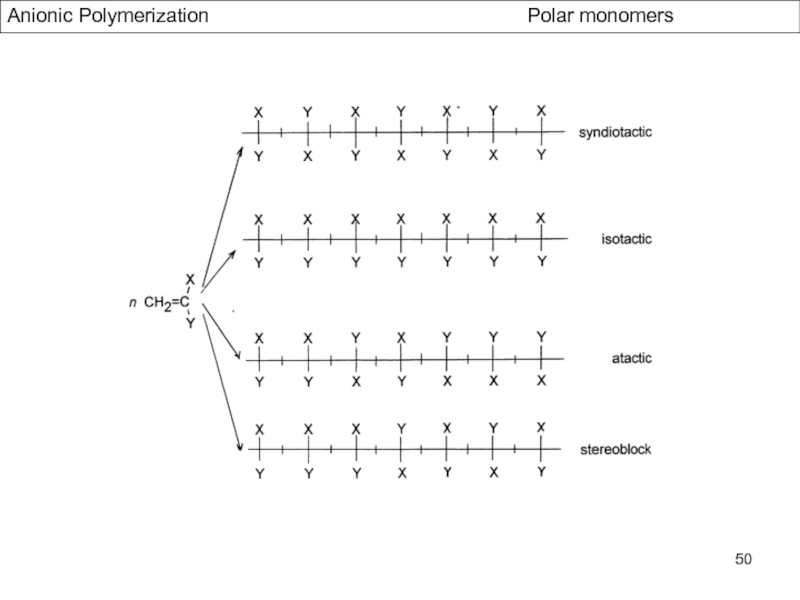
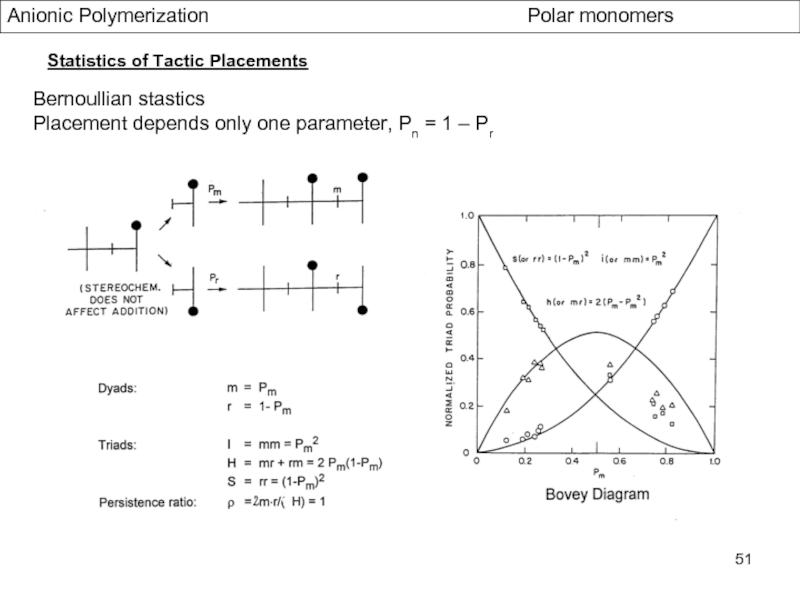
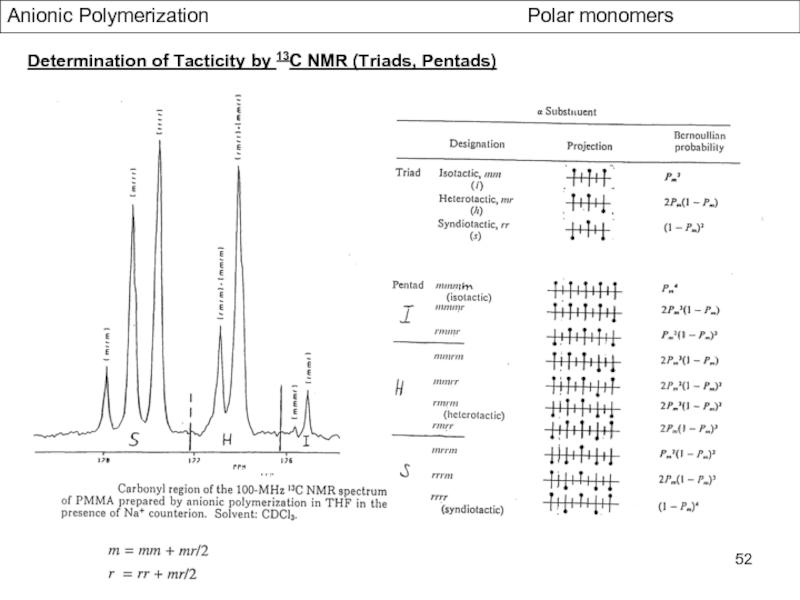
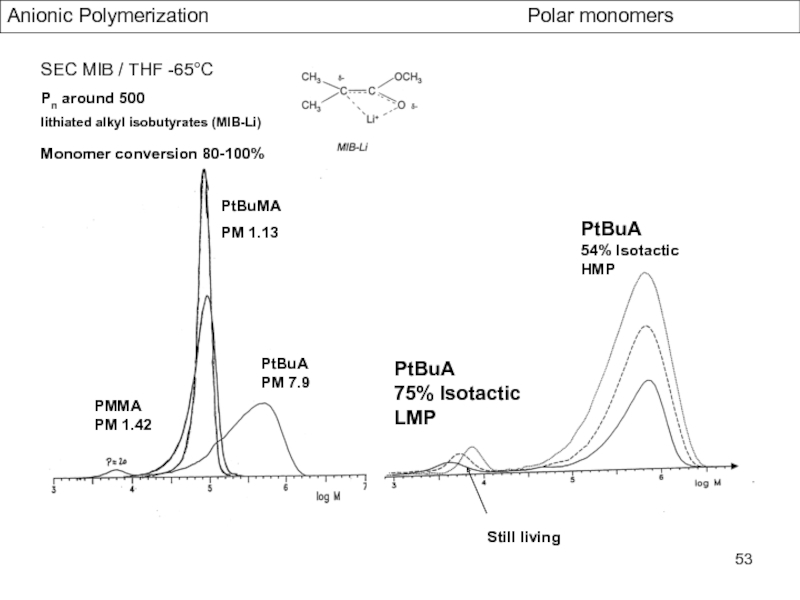
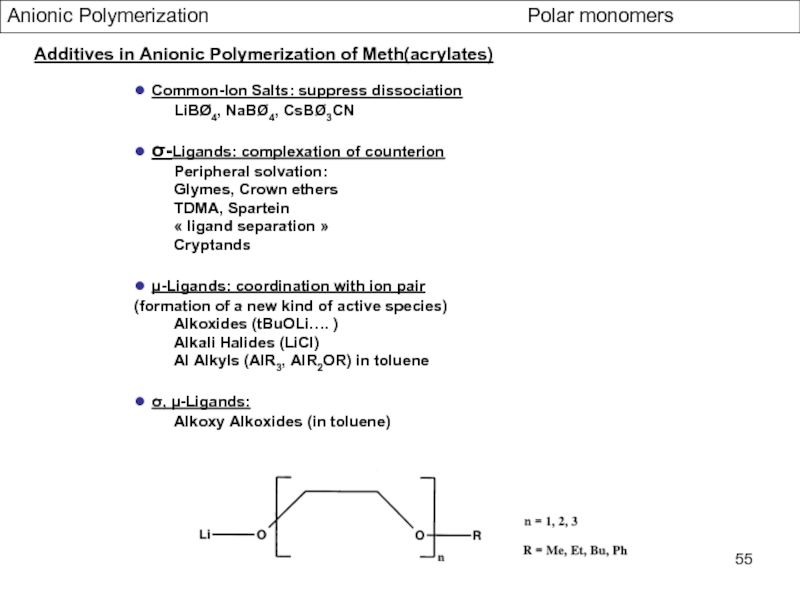
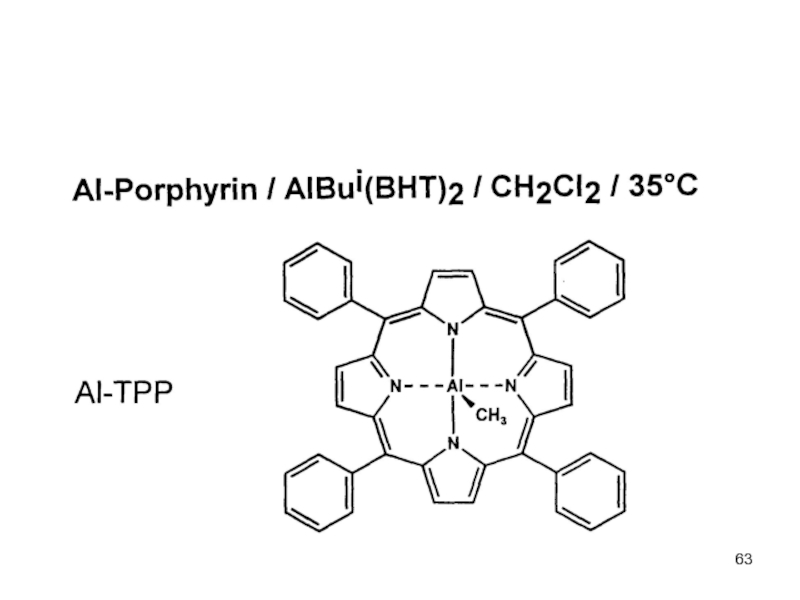
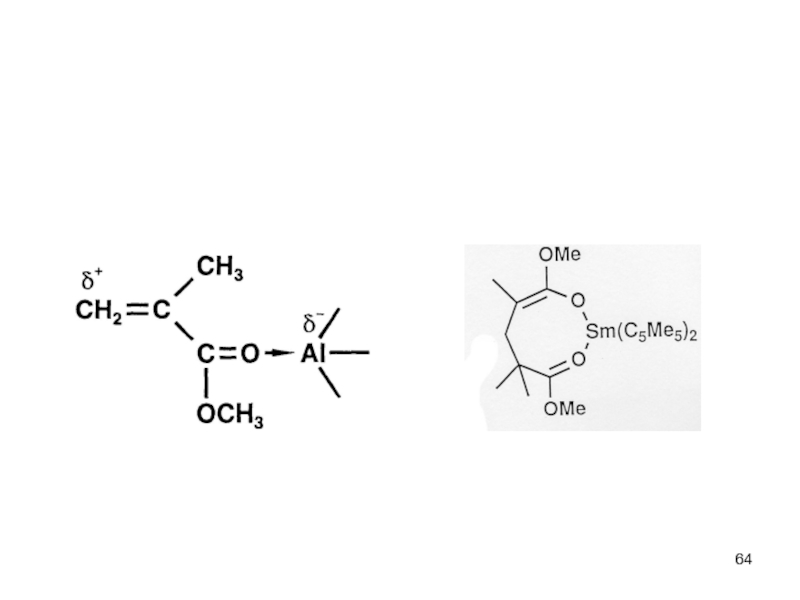
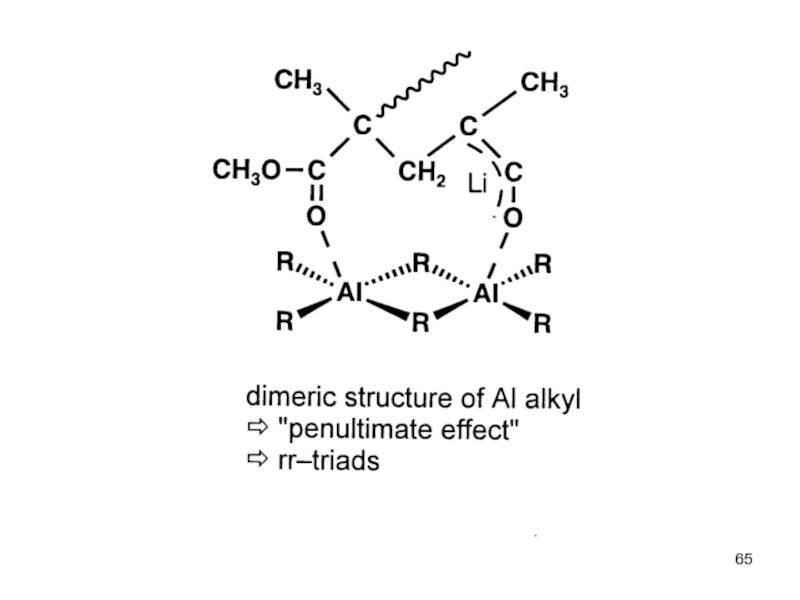
Control of Microstructure : Methods and distribution of tacticity
2 Anionic Polymerization of Non-polar Monomers
- Initiation and Propagation in Polar Solvents
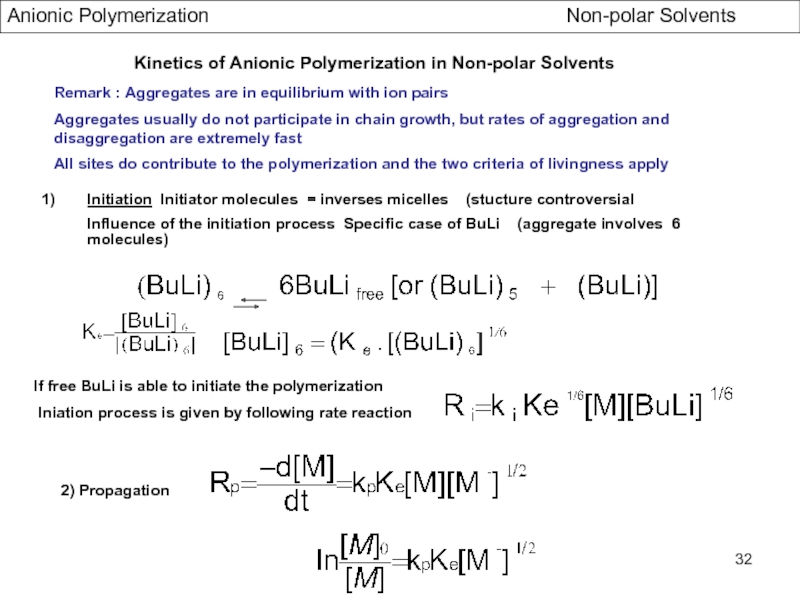
- Initiation and Propagation in Hydrocarbon Solvents
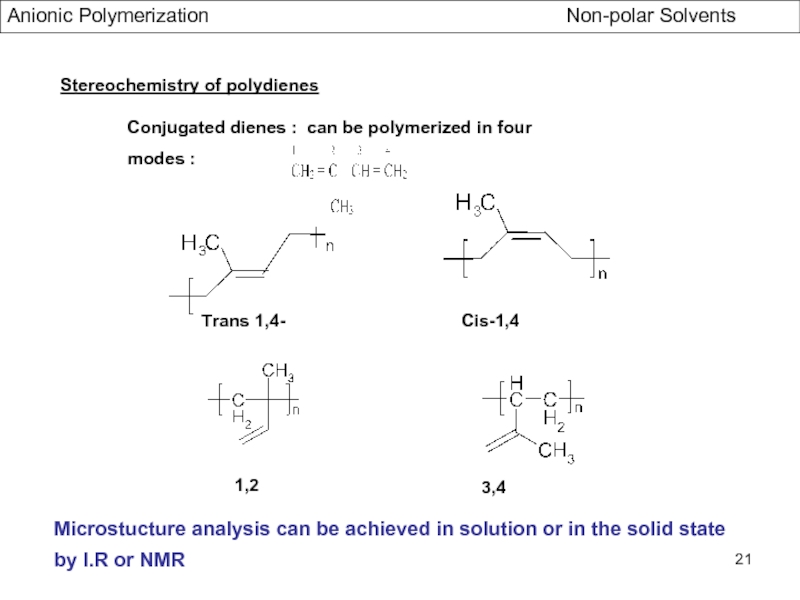
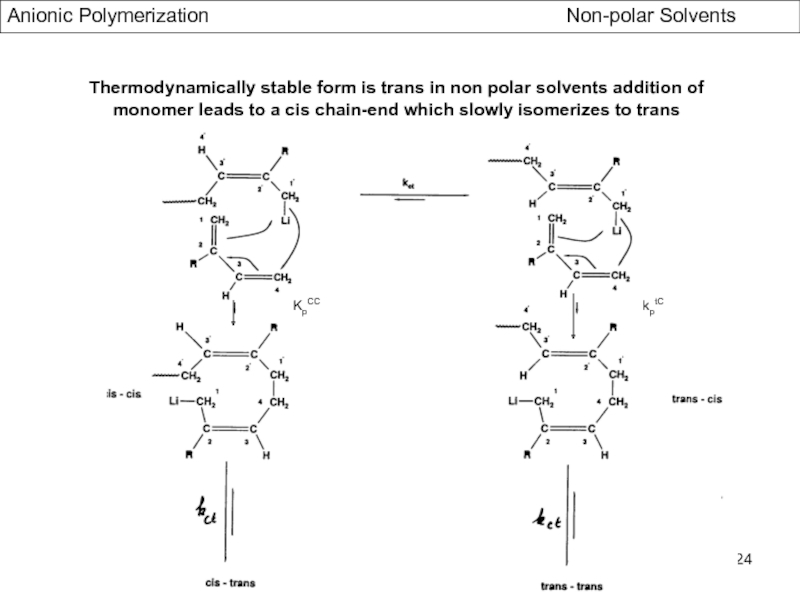
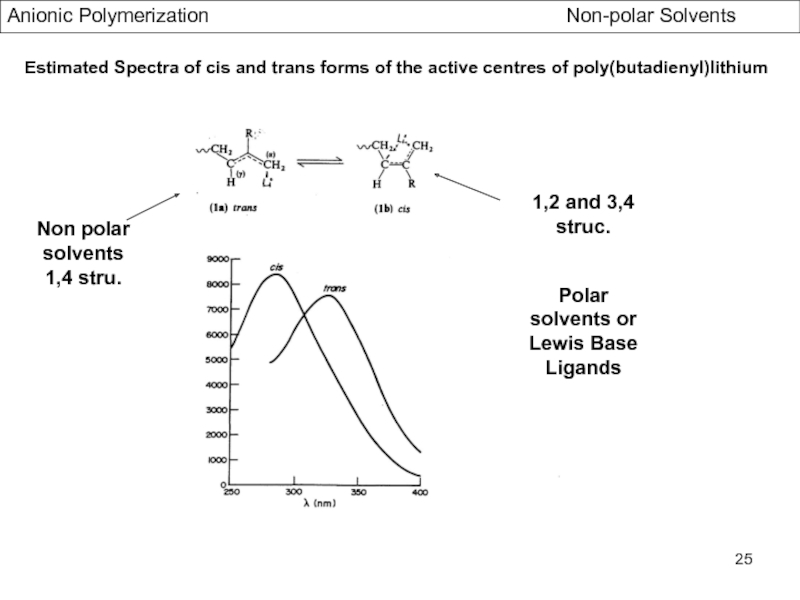
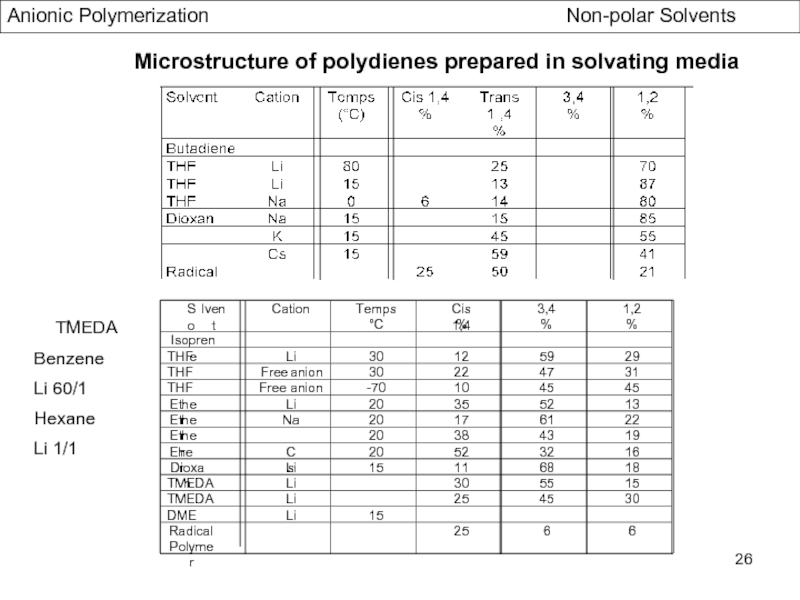
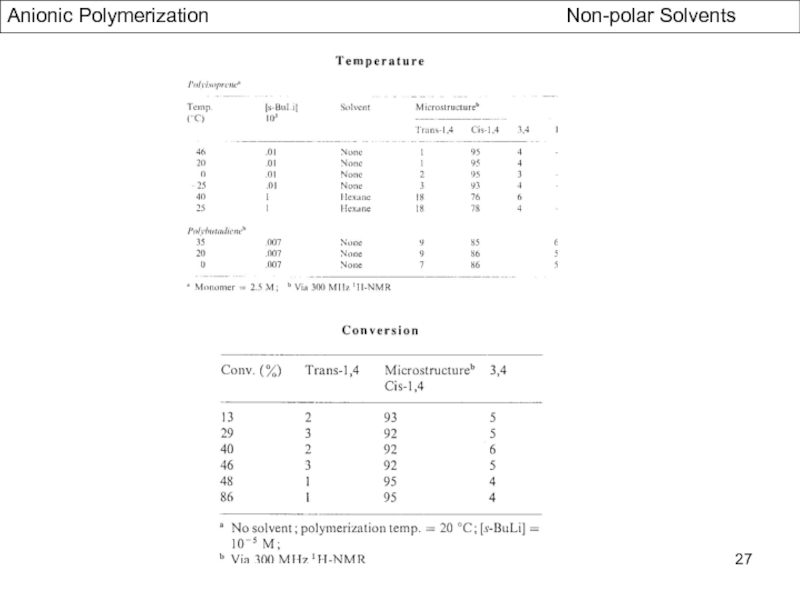
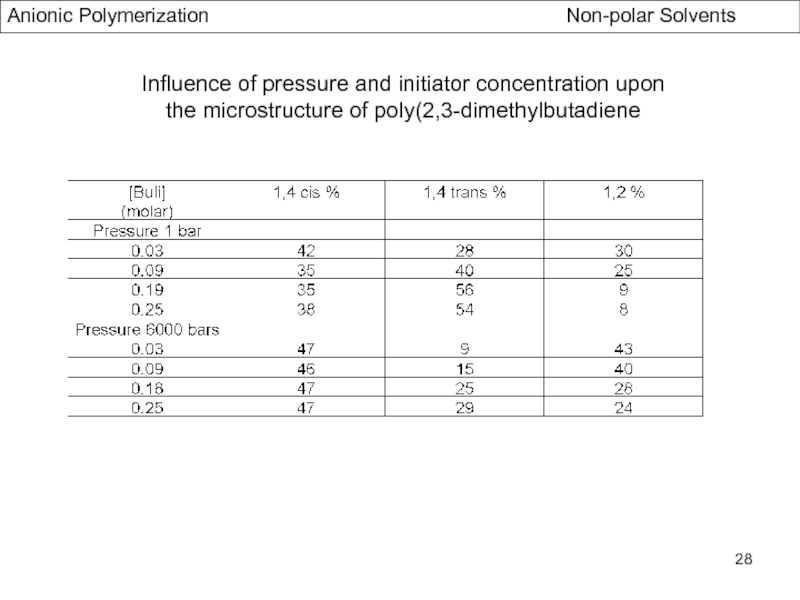
- Stereochemistry of Polydienes
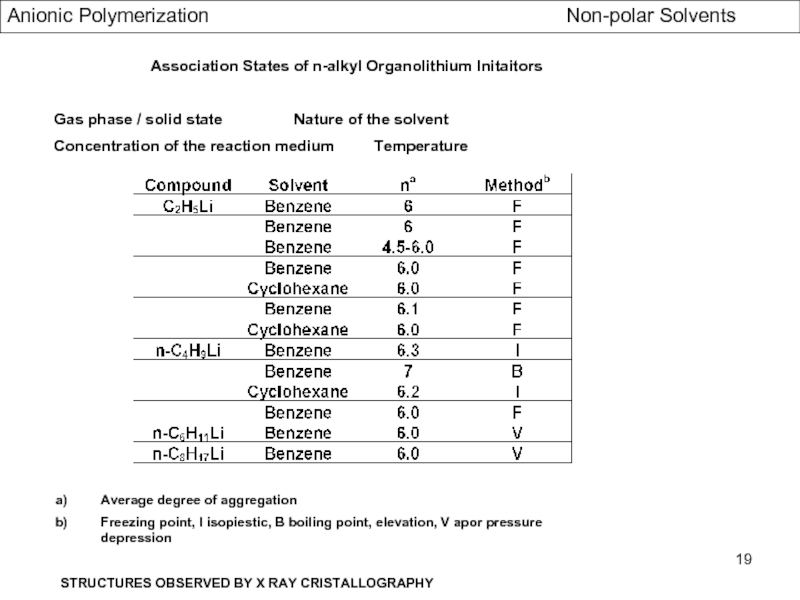
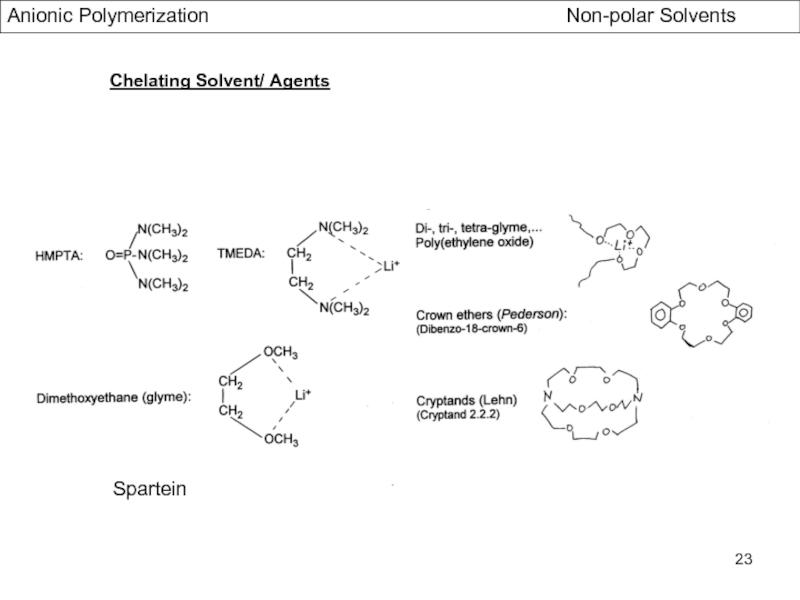
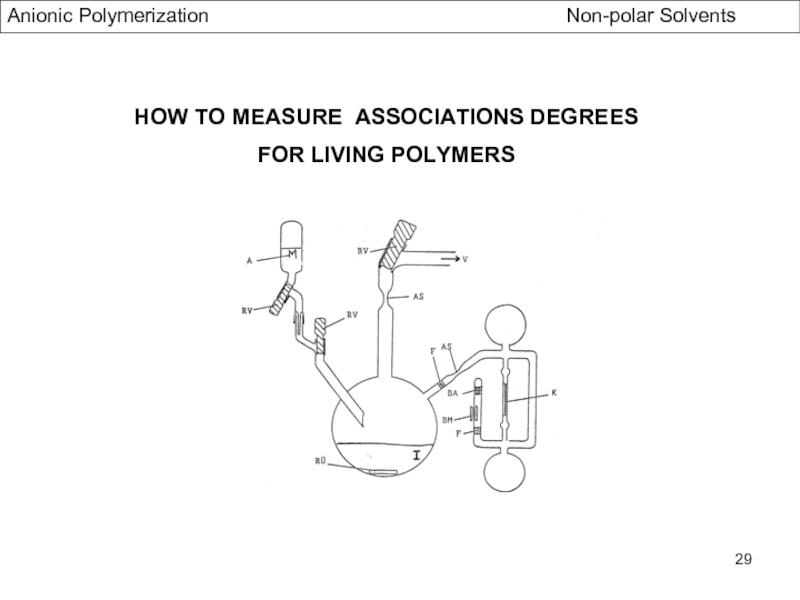
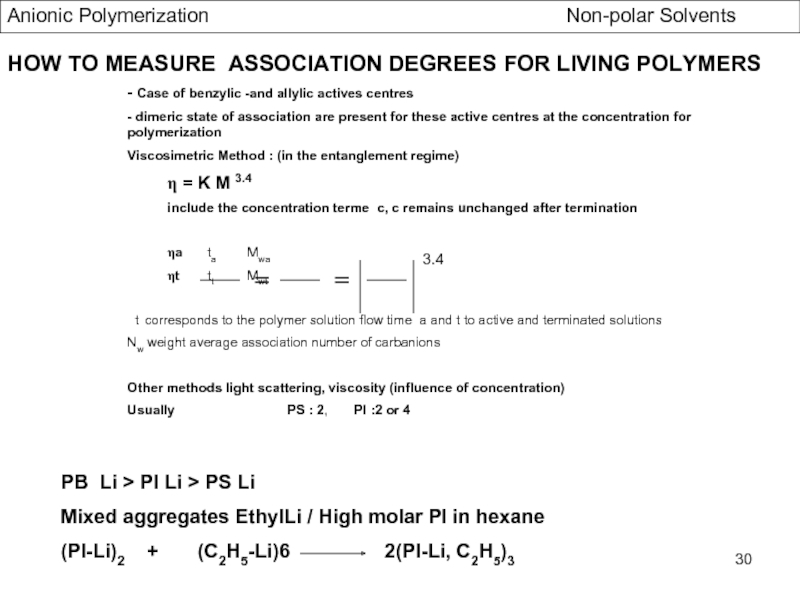
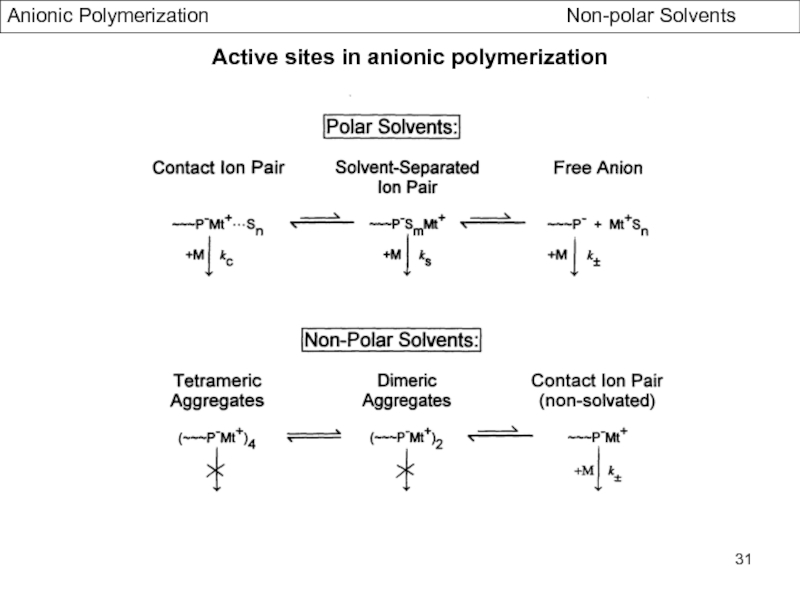
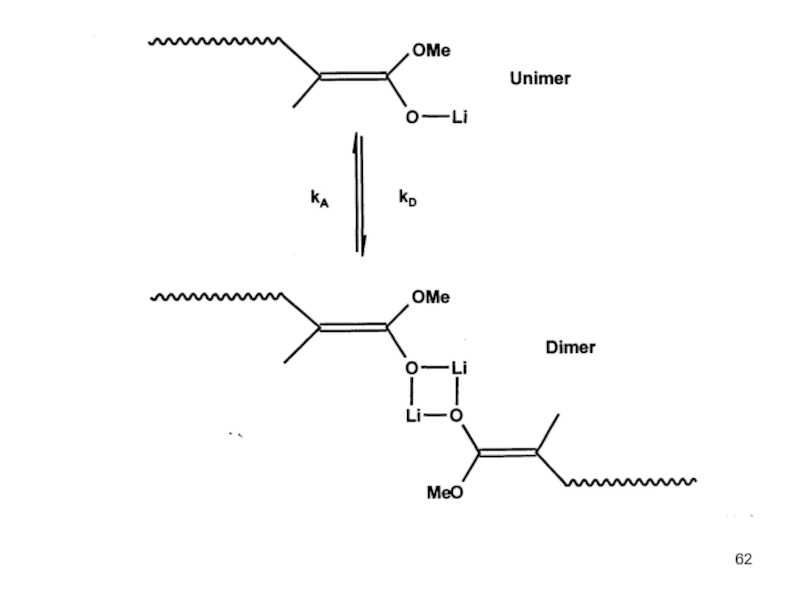
- Association Phenomena in non Polar Solvents
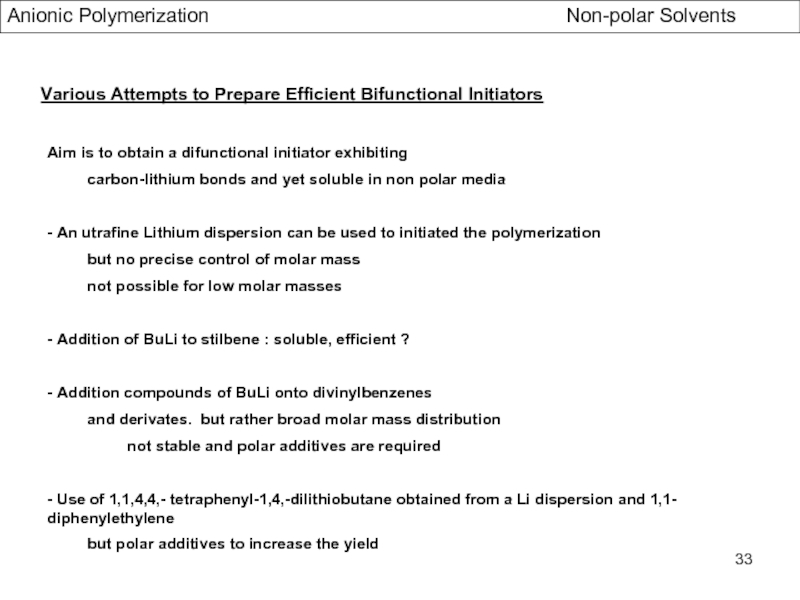
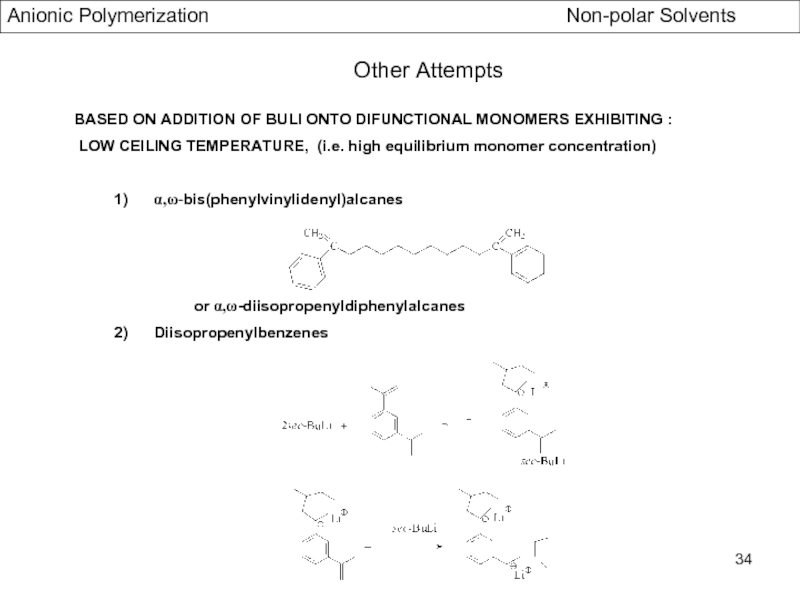
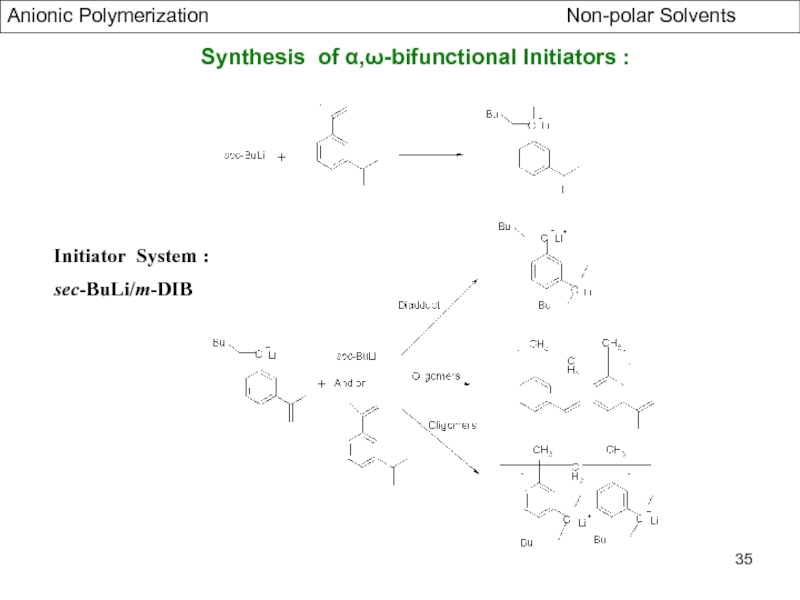
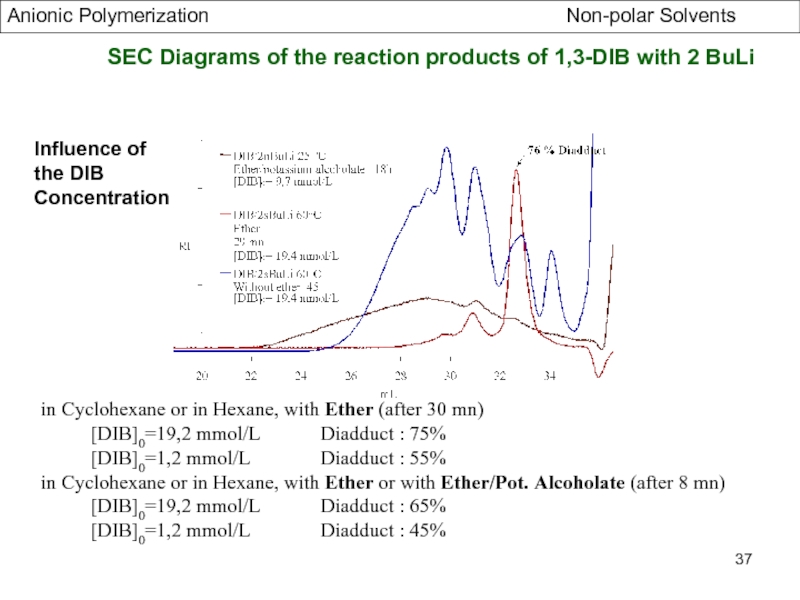
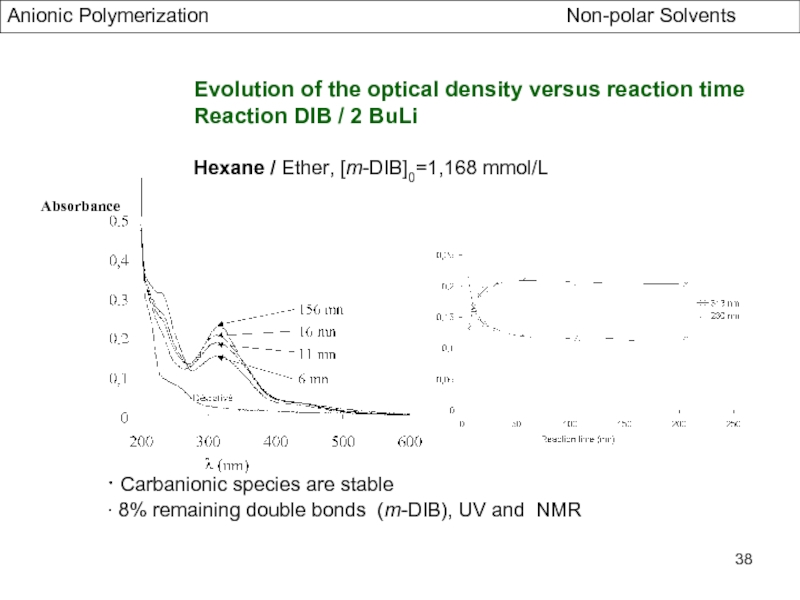
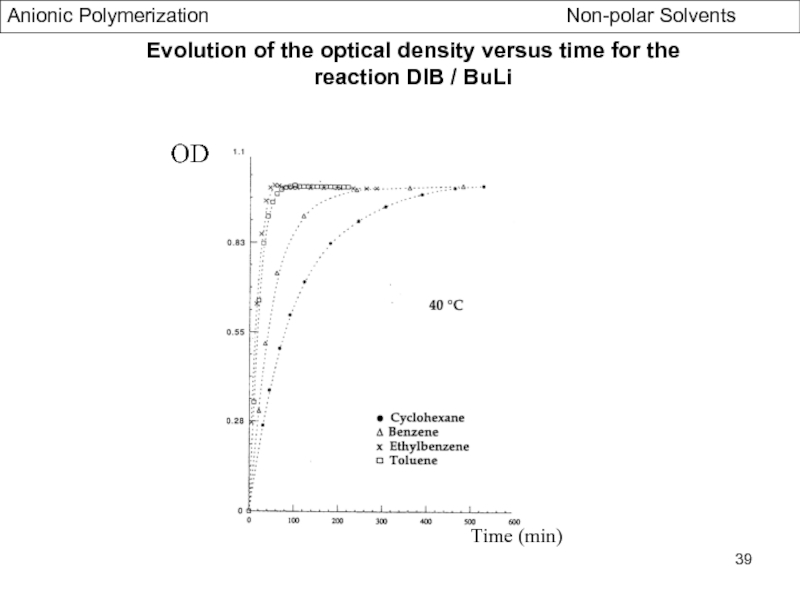
Specific Problems of bifunctional Initiators
Anionic Polymerization