- Fibrous proteins: building blocks
- Membrane proteins: transmitters
- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Fibrous proteins and their functions. Membrane proteins and their functions презентация
Содержание
- 1. Fibrous proteins and their functions. Membrane proteins and their functions
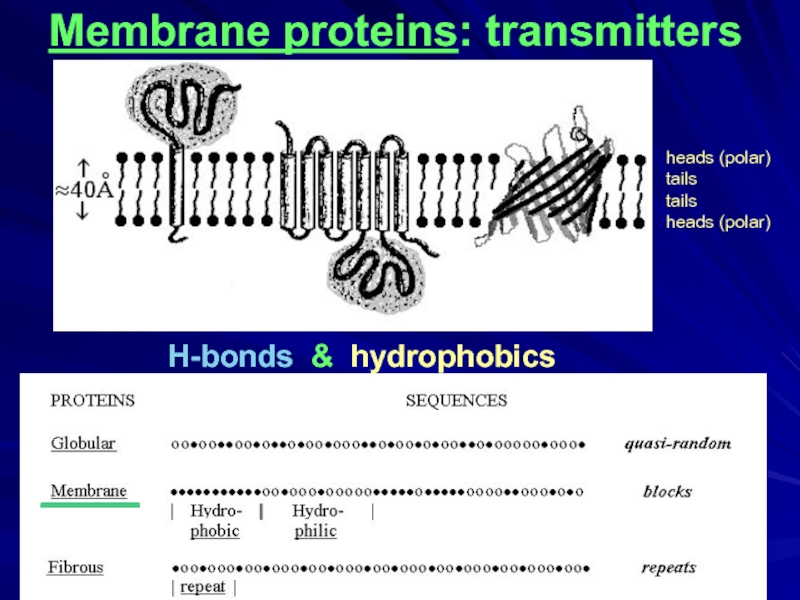
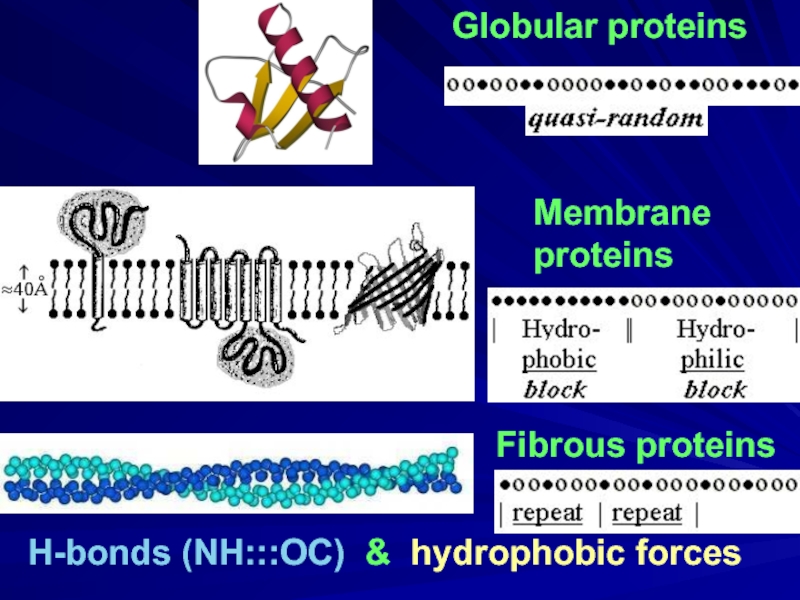
- 2. Globular proteins Fibrous proteins H-bonds (NH:::OC) & hydrophobic forces Membrane proteins
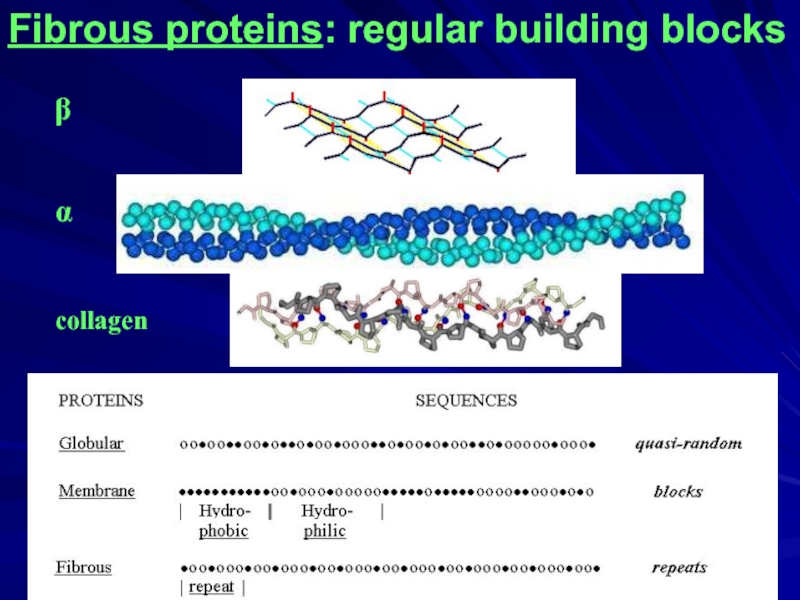
- 3. Fibrous proteins: regular building blocks ____________________________________
- 4. Fibrous proteins: regular building blocks β α collagen
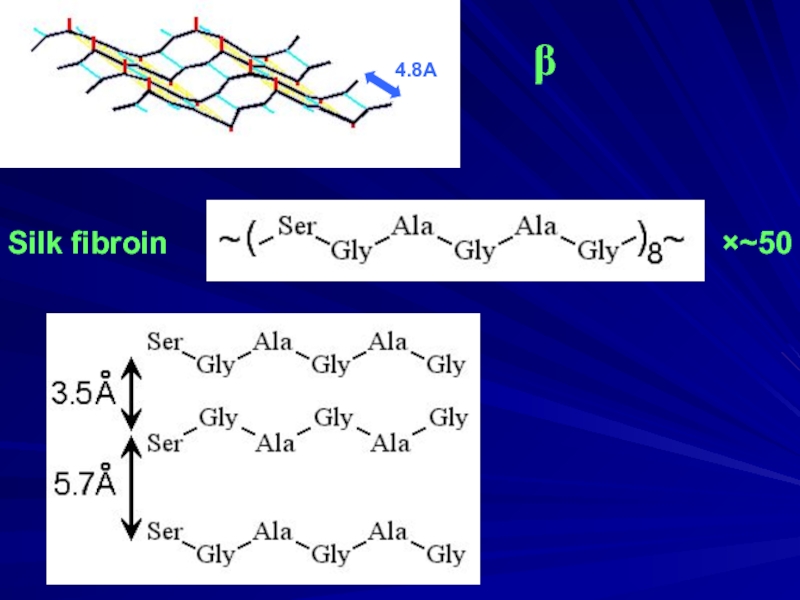
- 5. Silk fibroin ×~50 β 4.8A
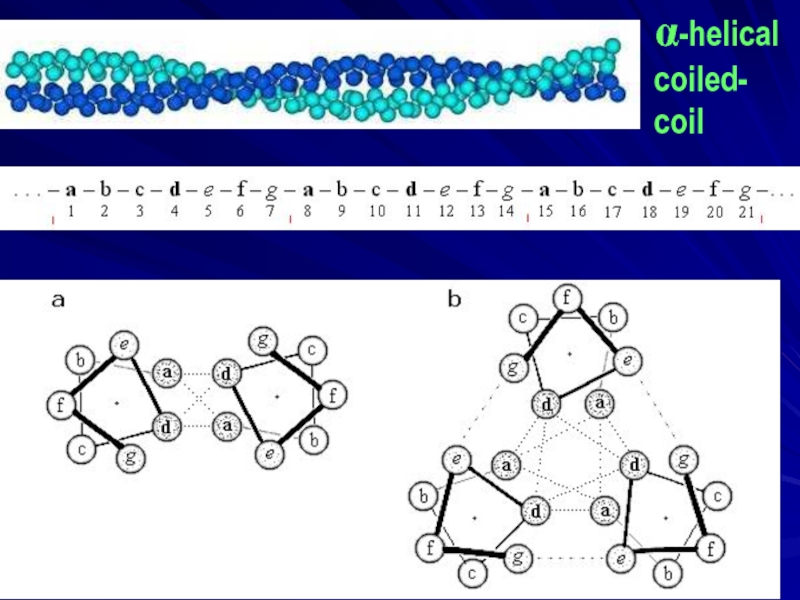
- 6. α-helical coiled- coil
- 7. Francis Harry Compton Crick (1916 – 2004) Nobel Prize
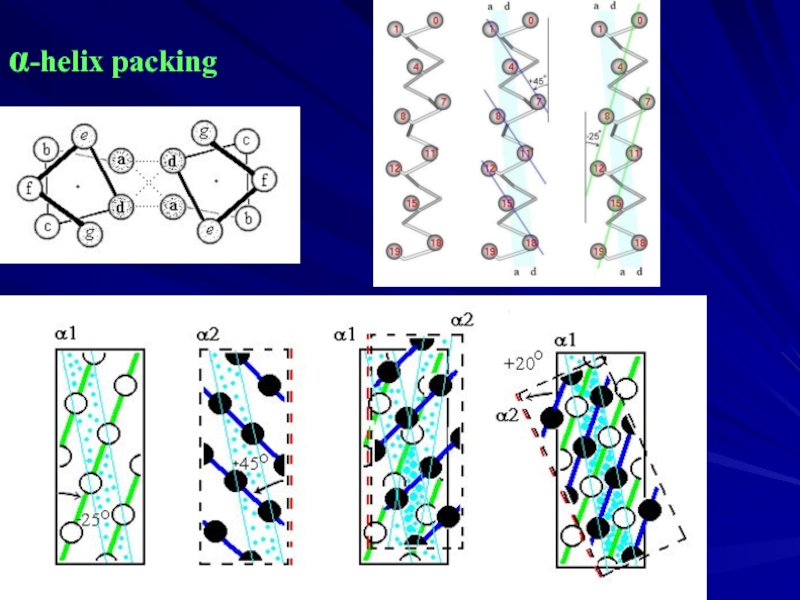
- 8. α-helix packing
- 9. collagen triple helix: 3 chains ≈ [Gly-X-Pro]≈500
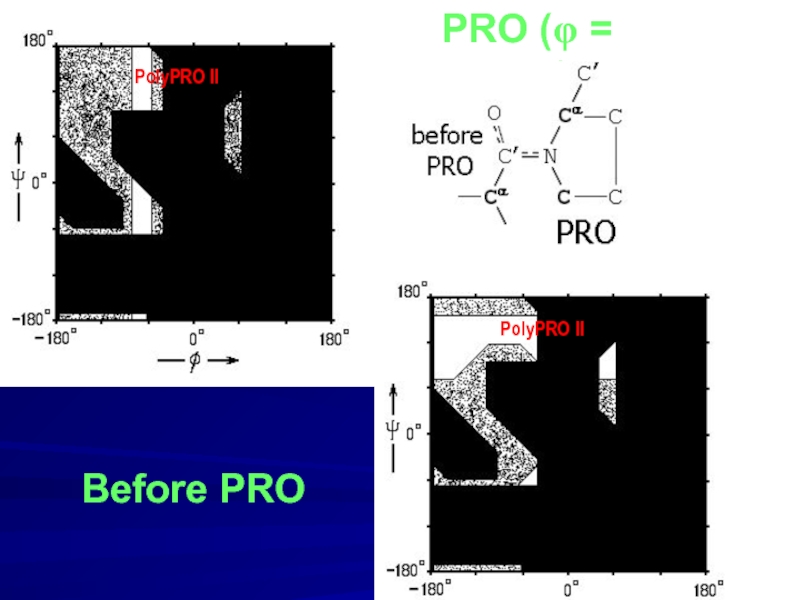
- 10. PRO (φ = -70o) Before PRO PolyPRO II PolyPRO II
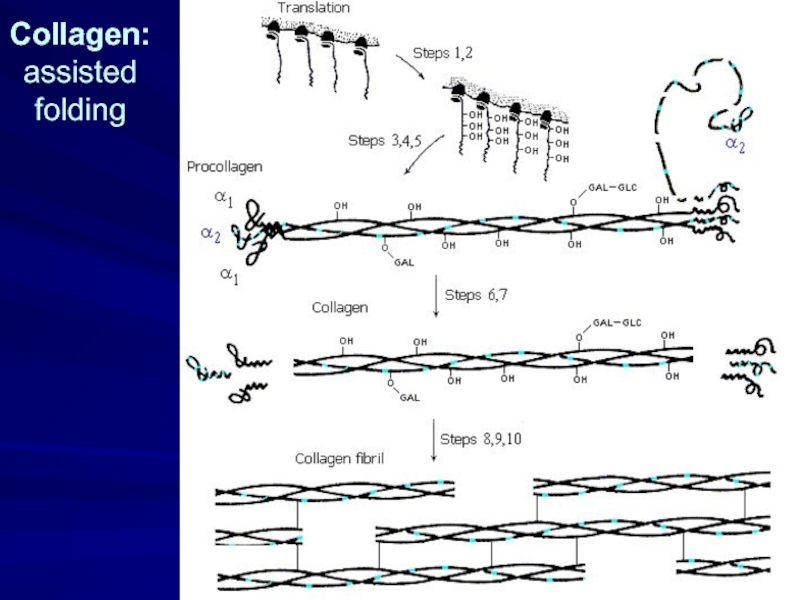
- 11. Collagen: assisted folding
- 12. Kuru: a mysterious disease, later demonstrated to
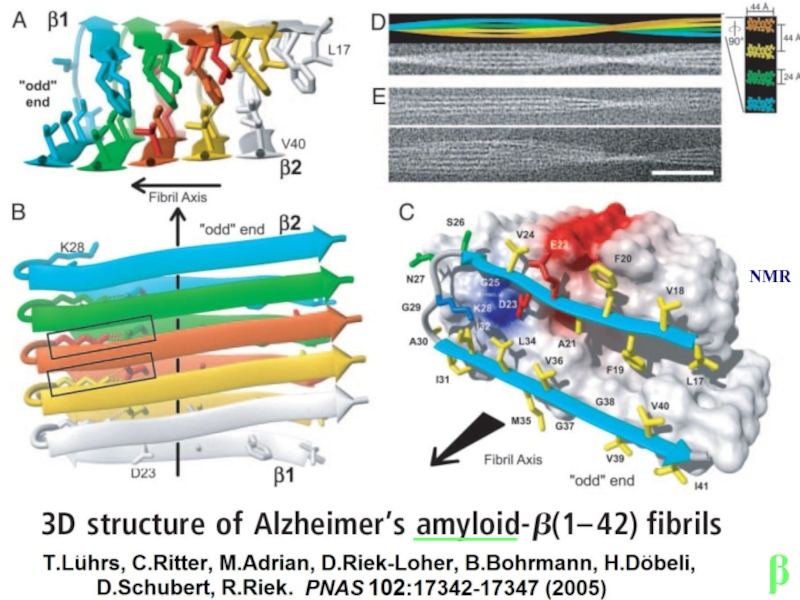
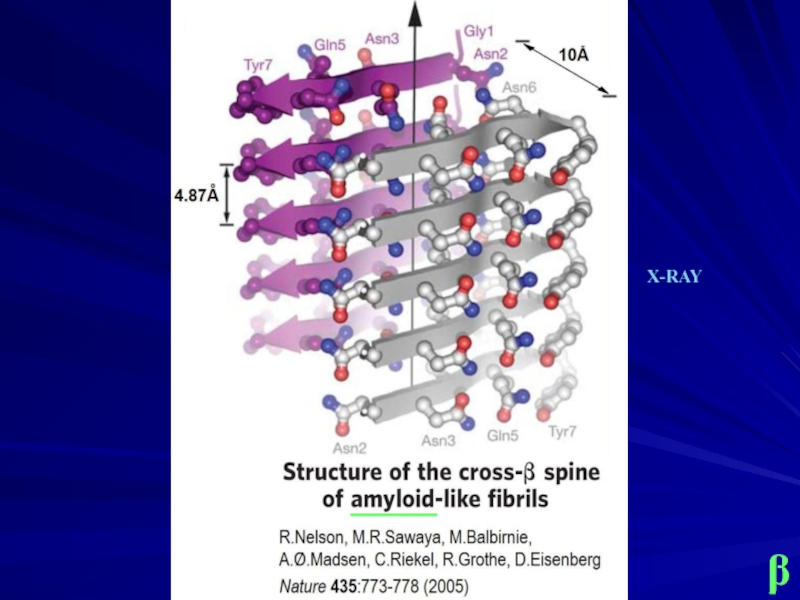
- 13. β ______ NMR
- 14. Lu J.X., Qiang W., Yau W.M.,
- 15. β _____ X-RAY
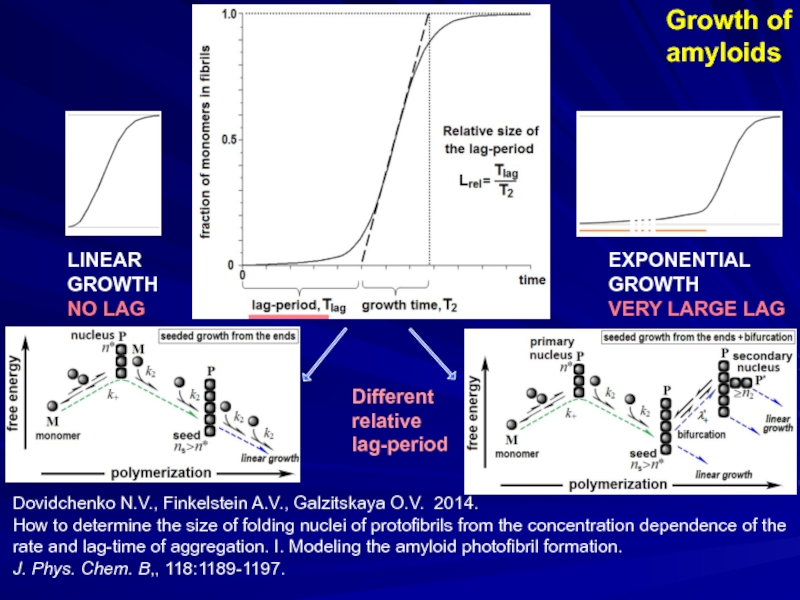
- 17. Growth of amyloids Dovidchenko N.V.,
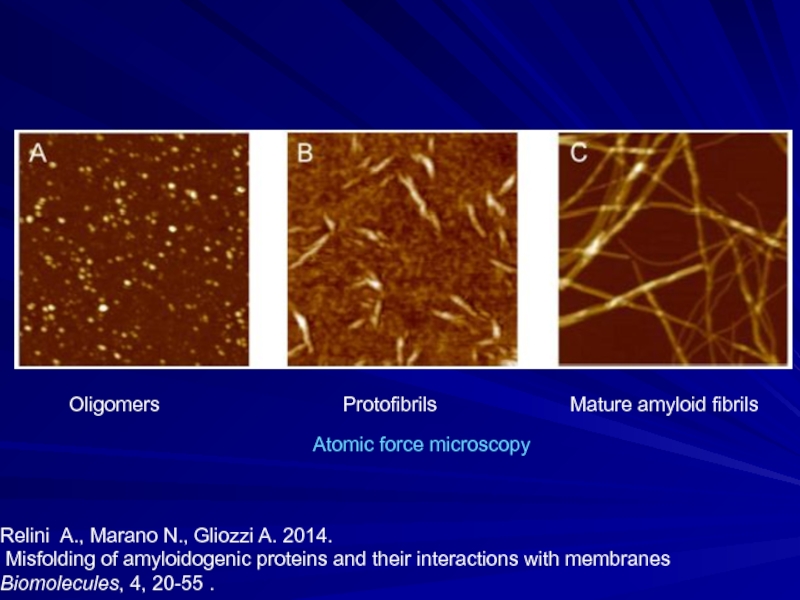
- 18. Oligomers
- 19. Elastin: Matrix protein. Short
- 20. H-bonds & hydrophobics Membrane proteins: transmitters ____ heads (polar) tails tails heads (polar)
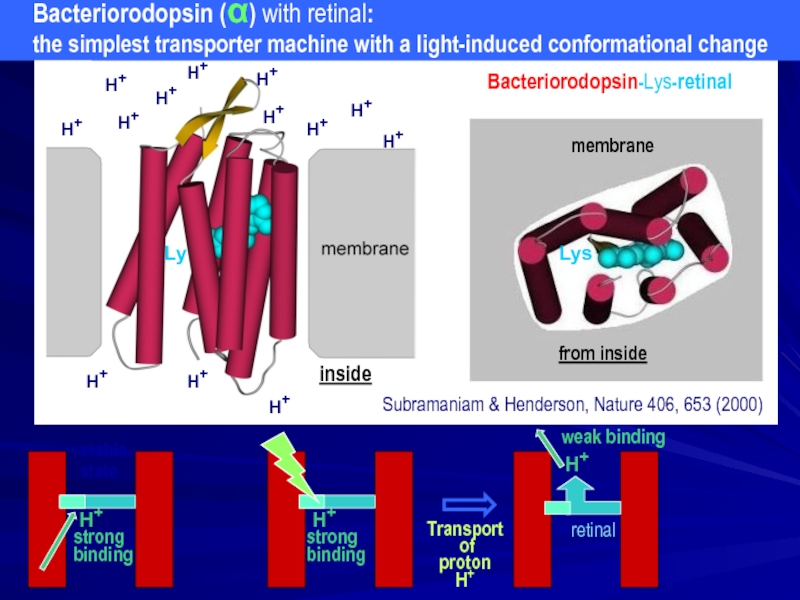
- 21. H+
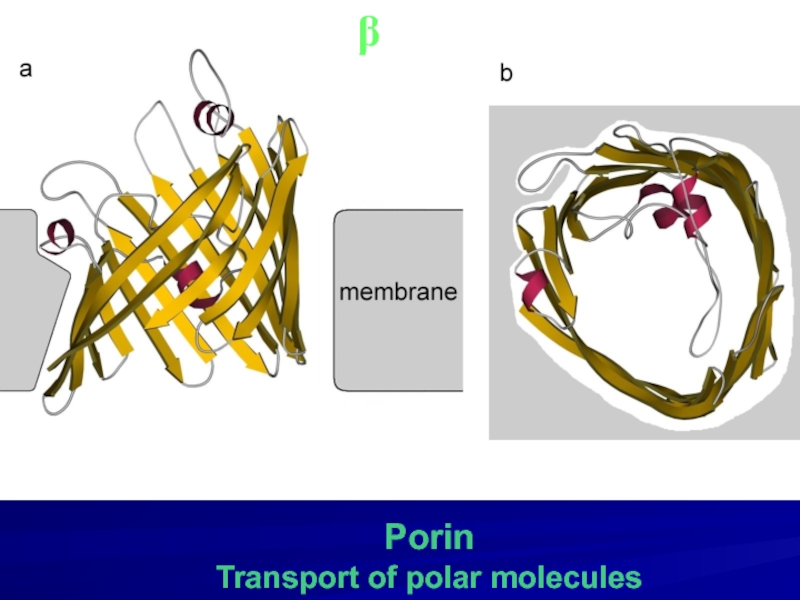
- 22. Porin Transport of polar molecules β
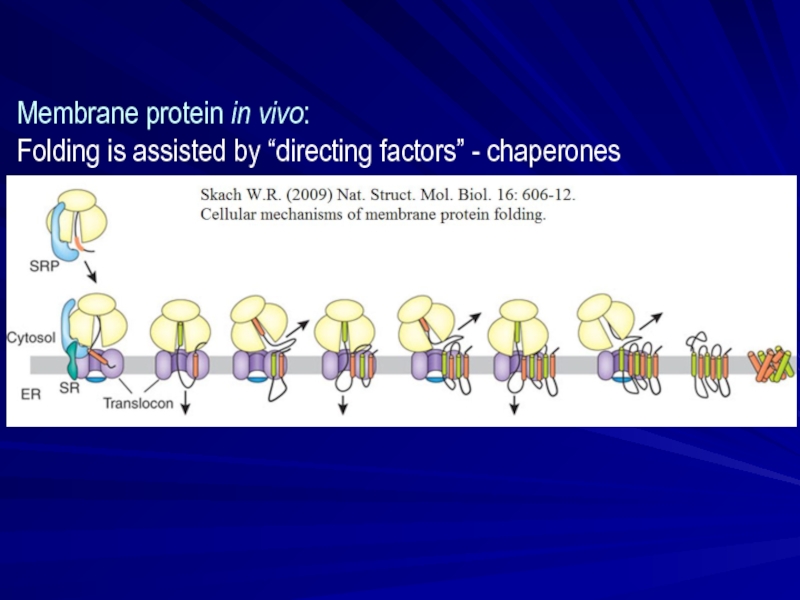
- 23. Membrane protein in vivo: Folding is assisted by “directing factors” - chaperones
- 24. MANY OF SIMPLE MEMBRANE PROTEINS REFOLD IN
- 25. Pore in membrane: SELECTIVITY Free energy
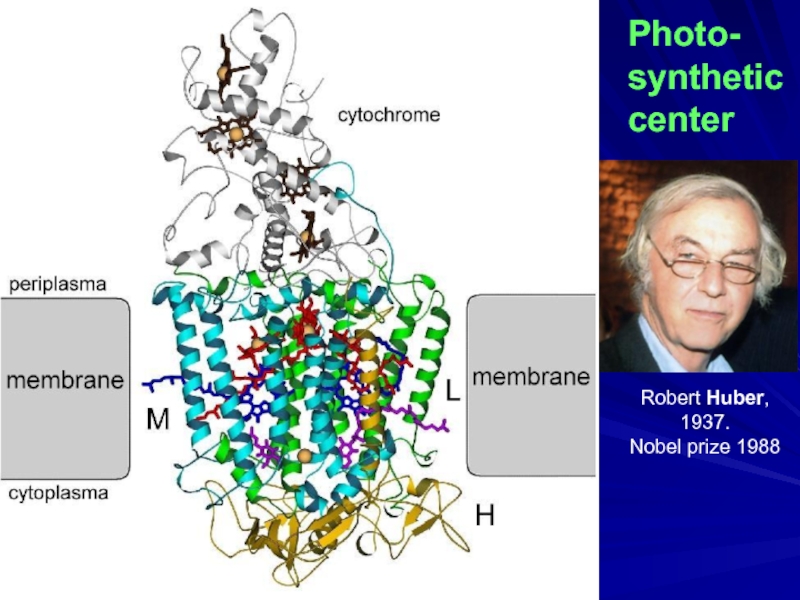
- 26. Photo- synthetic center Robert Huber, 1937. Nobel prize 1988
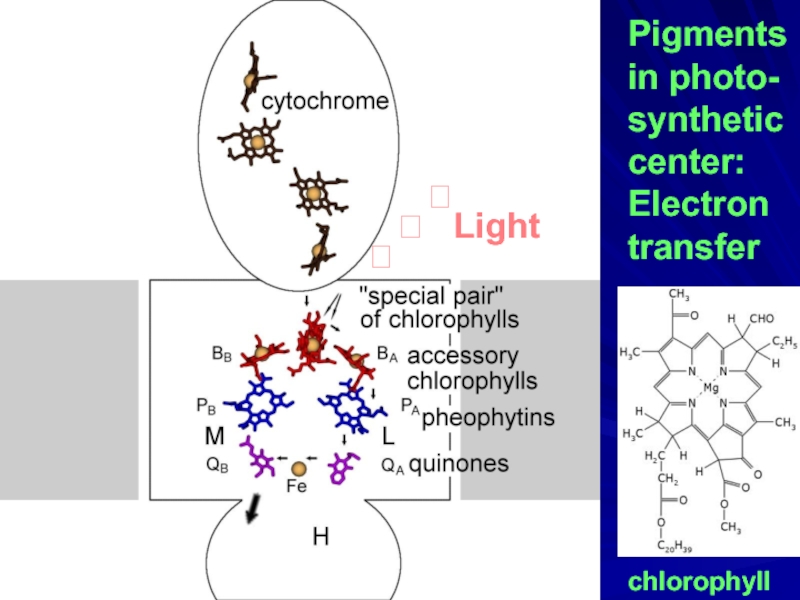
- 27. Pigments in photo- synthetic center: Electron transfer
- 28. Tunneling P(X) ~ 10-X(Å) T-independent Frequency
Слайд 1
PROTEIN PHYSICS
LECTURES 11-12
- Fibrous proteins and their functions
- Membrane proteins and
Слайд 3
Fibrous proteins: regular building blocks
____________________________________
Here, we will not consider fibrous proteins
made of globules (actin, etc.)
β
α
collagen
Слайд 7Francis Harry Compton Crick (1916 – 2004)
Nobel Prize 1962
for DNA structure, 1953
C. Chothia, M. Levitt, D. Richardson, 1977
Слайд 12Kuru: a mysterious disease, later demonstrated to be infectious prion disease.
Daniel
Baruch Samuel Blumberg (1925 – 2011)
Nobel Prize 1976
PRION: PROtein and Infection
Stanley Benjamin Prusiner, 1942
Nobel Prize 1997
Studies of amyloid formation
Christopher Martin Dobson, 1949
Royal Medal 2009
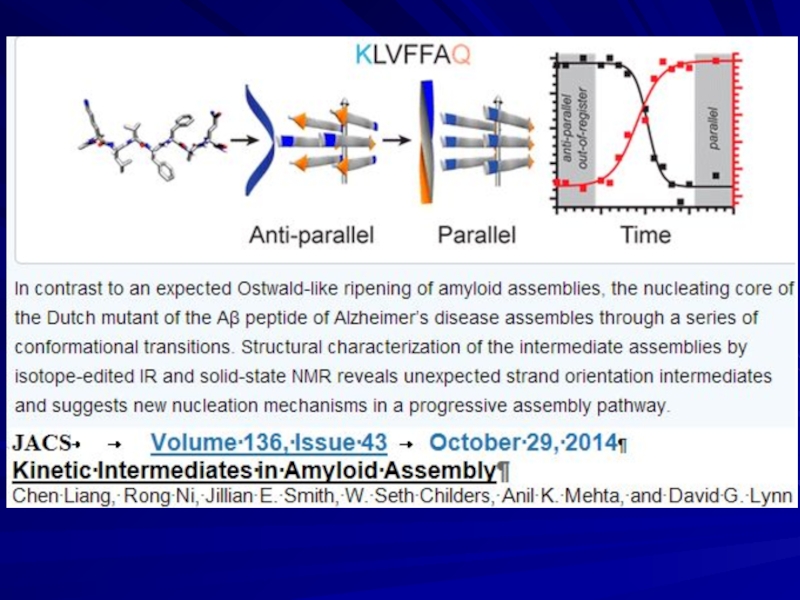
Слайд 14
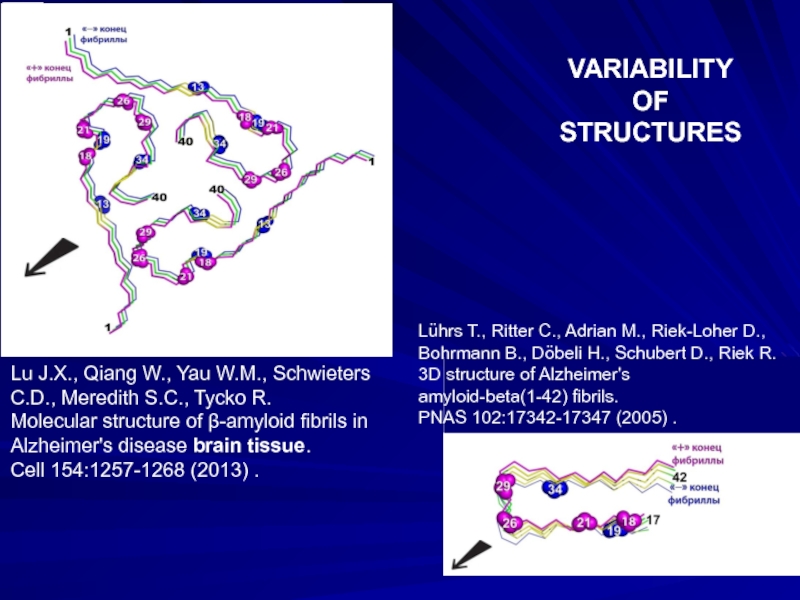
Lu J.X., Qiang W., Yau W.M., Schwieters C.D., Meredith S.C., Tycko
Molecular structure of β-amyloid fibrils in Alzheimer's disease brain tissue.
Cell 154:1257-1268 (2013) .
Lührs T., Ritter C., Adrian M., Riek-Loher D., Bohrmann B., Döbeli H., Schubert D., Riek R.
3D structure of Alzheimer's
amyloid-beta(1-42) fibrils.
PNAS 102:17342-17347 (2005) .
VARIABILITY
OF
STRUCTURES
Слайд 17
Growth of
amyloids
Dovidchenko N.V., Finkelstein A.V., Galzitskaya O.V. 2014.
How to determine
J. Phys. Chem. B,, 118:1189-1197.
LINEAR
GROWTH
NO LAG
EXPONENTIAL
GROWTH
VERY LARGE LAG
Different
relative
lag-period
Слайд 18 Oligomers
Relini A., Marano N., Gliozzi A. 2014.
Misfolding of amyloidogenic proteins and their interactions with membranes
Biomolecules, 4, 20-55 .
Atomic force microscopy
Слайд 19Elastin:
Matrix protein.
Short repeats.
Poor secondary structure.
Chains are linked by
modified Lys residues.
Like in rubber.
Natively non-structured fibrous proteins:
Слайд 20
H-bonds & hydrophobics
Membrane proteins: transmitters
____
heads (polar)
tails
tails
heads (polar)
Слайд 21
H+
strong
binding
H+
inside
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
H+
weak binding
H+
strong
binding
stable
state
Transport
proton
H+
Bacteriorodopsin-Lys-retinal
from inside
membrane
Subramaniam & Henderson, Nature 406, 653 (2000)
Lys
Ly
Bacteriorodopsin (α) with retinal:
the simplest transporter machine with a light-induced conformational change
retinal
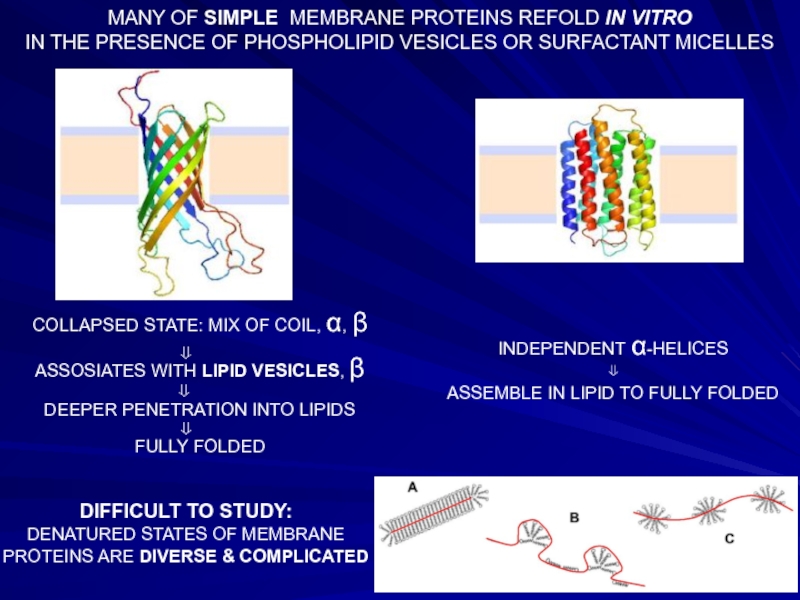
Слайд 24MANY OF SIMPLE MEMBRANE PROTEINS REFOLD IN VITRO
IN THE PRESENCE OF
COLLAPSED STATE: MIX OF COIL, α, β
ASSOSIATES WITH LIPID VESICLES, β
DEEPER PENETRATION INTO LIPIDS
FULLY FOLDED
INDEPENDENT α-HELICES
⇓
ASSEMBLE IN LIPID TO FULLY FOLDED
⇓
⇓
⇓
DIFFICULT TO STUDY:
DENATURED STATES OF MEMBRANE PROTEINS ARE DIVERSE & COMPLICATED
Слайд 25Pore in membrane: SELECTIVITY
Free energy of a charge in the non-charged
~ q2 / [(εMEMBR εWATER )1/2 rPORE] ~
~ 20 kcal/mol / rPORE(Å)
+
Слайд 28Tunneling
P(X) ~ 10-X(Å)
T-independent
Frequency of vibrations (attacks):
f ~ 1015/sec
Successful attacks:
fSUCCS.(x) ~ P(x)•f,
fSUCCS.(5Å) ~ 10-5+15 ~
~ 1010/sec
Atom ≈ 1Å ⇒ Attenuation of
electron density:
≅
~
V = ±|V|



![collagen triple helix: 3 chains ≈ [Gly-X-Pro]≈500](/img/tmb/5/401970/6a125166a87ffd51a06e6244744a2f51-800x.jpg)