- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
The molecular basis of inheritance. (Chapter 16) презентация
Содержание
- 1. The molecular basis of inheritance. (Chapter 16)
- 2. Overview: Life’s Operating Instructions In 1953, James
- 3. Figure 16.1
- 4. Concept 16.1: DNA is the genetic material
- 5. The Search for the Genetic Material: Scientific
- 6. Evidence That DNA Can Transform Bacteria The
- 7. When he mixed heat-killed remains of
- 8. Living S cells (control) Living R cells
- 9. In 1944, Oswald Avery, Maclyn McCarty, and
- 10. Evidence That Viral DNA Can Program Cells
- 11. Animation: Phage T2 Reproductive Cycle Right-click slide / select “Play”
- 12. Figure 16.3 Phage head Tail sheath Tail fiber DNA Bacterial cell 100 nm
- 13. In 1952, Alfred Hershey and Martha Chase
- 14. Animation: Hershey-Chase Experiment Right-click slide / select “Play”
- 15. Figure 16.4-1 Bacterial cell Phage Batch 1:
- 16. Figure 16.4-2 Bacterial cell Phage Batch 1:
- 17. Figure 16.4-3 Bacterial cell Phage Batch 1:
- 18. Additional Evidence That DNA Is the Genetic
- 19. Animation: DNA and RNA Structure Right-click slide / select “Play”
- 20. Two findings became known as Chargaff’s rules
- 21. Figure 16.5 Sugar–phosphate backbone Nitrogenous bases Thymine
- 22. Building a Structural Model of DNA: Scientific
- 23. Figure 16.6 (a) Rosalind Franklin
- 24. Figure 16.6a (a) Rosalind Franklin
- 25. Figure 16.6b
- 26. Franklin’s X-ray crystallographic images of DNA enabled
- 27. Animation: DNA Double Helix Right-click slide / select “Play”
- 28. Figure 16.7 3.4 nm 1 nm 0.34
- 29. 3.4 nm 1 nm 0.34 nm Hydrogen
- 30. Figure 16.7b (c) Space-filling model
- 31. Watson and Crick built models of a
- 32. At first, Watson and Crick thought the
- 33. Figure 16.UN01 Purine + purine: too wide
- 34. Watson and Crick reasoned that the pairing
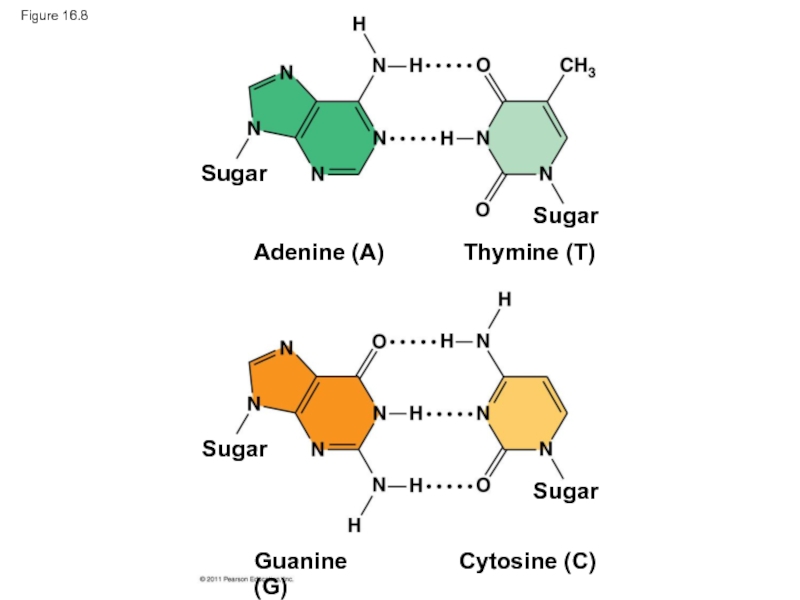
- 35. Figure 16.8 Sugar Sugar Sugar Sugar Adenine (A) Thymine (T) Guanine (G) Cytosine (C)
- 36. Concept 16.2: Many proteins work together in
- 37. The Basic Principle: Base Pairing to a
- 38. Animation: DNA Replication Overview Right-click slide / select “Play”
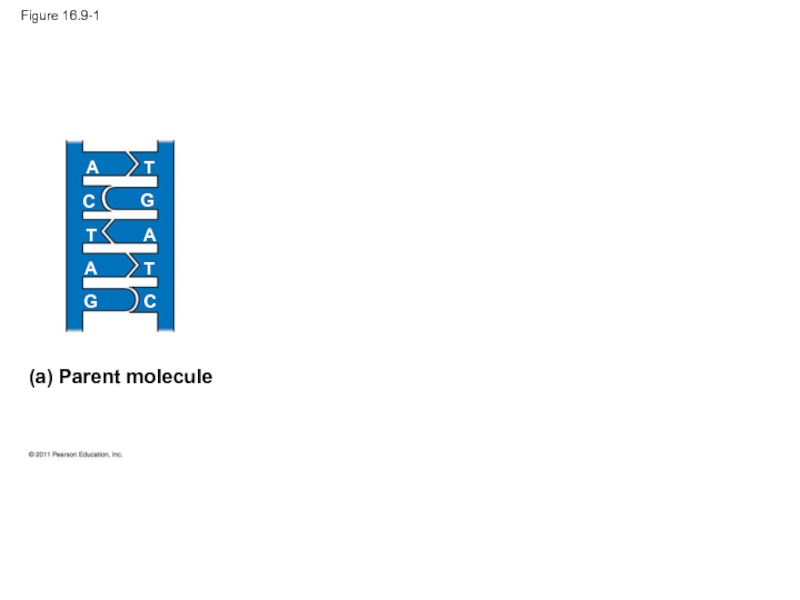
- 39. Figure 16.9-1 (a) Parent molecule A A A T T T C C G G
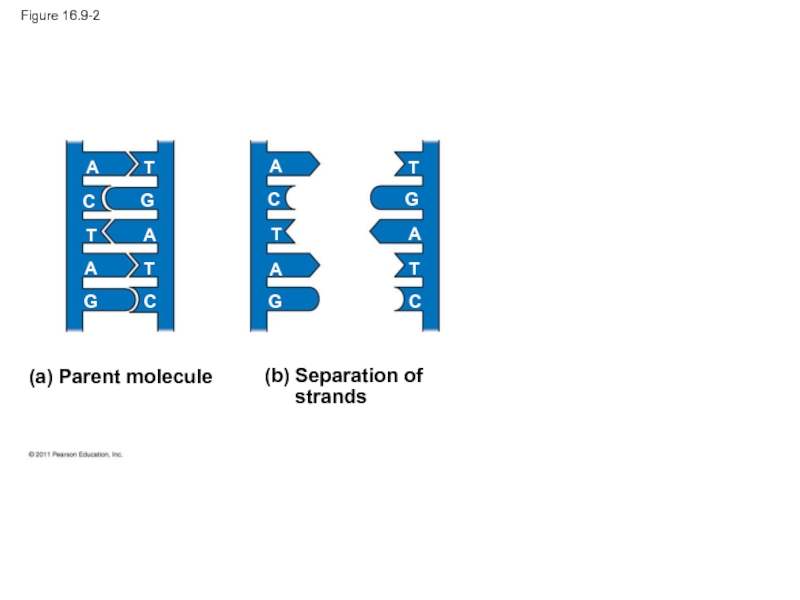
- 40. Figure 16.9-2 (a) Parent molecule A A
- 41. Figure 16.9-3 (a) Parent molecule A A
- 42. Watson and Crick’s semiconservative model of replication
- 43. Figure 16.10 (c) Dispersive model Parent cell First replication Second replication
- 44. Experiments by Matthew Meselson and Franklin Stahl
- 45. The first replication produced a band of
- 46. Figure 16.11 Bacteria cultured in medium with
- 47. Figure 16.11a Bacteria cultured in medium with
- 48. Figure 16.11b Predictions: First replication Second replication Conservative model Semiconservative model Dispersive model CONCLUSION
- 49. DNA Replication: A Closer Look The copying
- 50. Getting Started Replication begins at particular sites
- 51. Animation: Origins of Replication Right-click slide / select “Play”
- 52. Figure 16.12 (a) Origin of replication in
- 53. Figure 16.12a (a) Origin of replication in
- 54. Figure 16.12b (b) Origins of replication in
- 55. Figure 16.12c 0.5 μm
- 56. Figure 16.12d 0.25 μm
- 57. At the end of each replication bubble
- 58. Figure 16.13 Topoisomerase Primase RNA primer Helicase
- 59. DNA polymerases cannot initiate synthesis of a
- 60. An enzyme called primase can start an
- 61. Synthesizing a New DNA Strand Enzymes called
- 62. Each nucleotide that is added to a
- 63. Figure 16.14 New strand Template strand Sugar
- 64. Antiparallel Elongation The antiparallel structure of the
- 65. Along one template strand of DNA, the
- 66. Animation: Leading Strand Right-click slide / select “Play”
- 67. Figure 16.15 Leading strand Lagging strand Overview
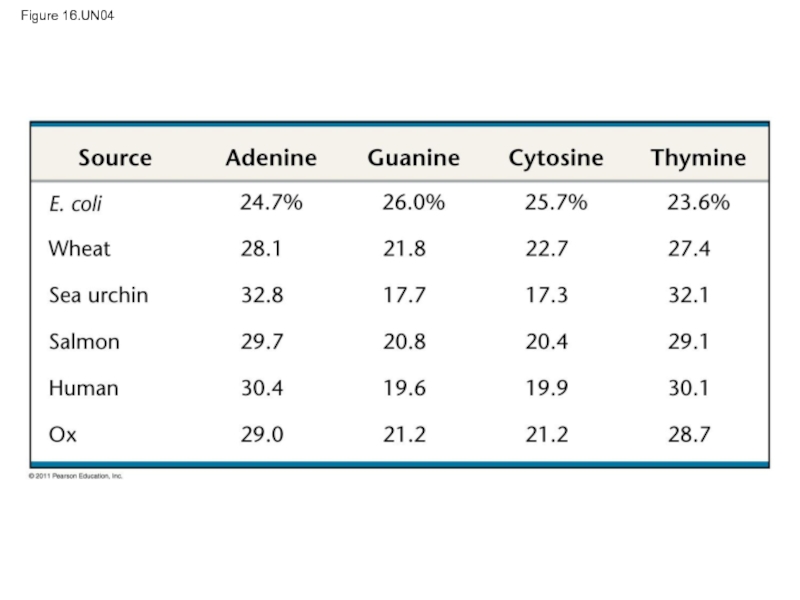
- 68. Figure 16.15a Leading strand Lagging strand Overview
- 69. Origin of replication RNA primer Sliding
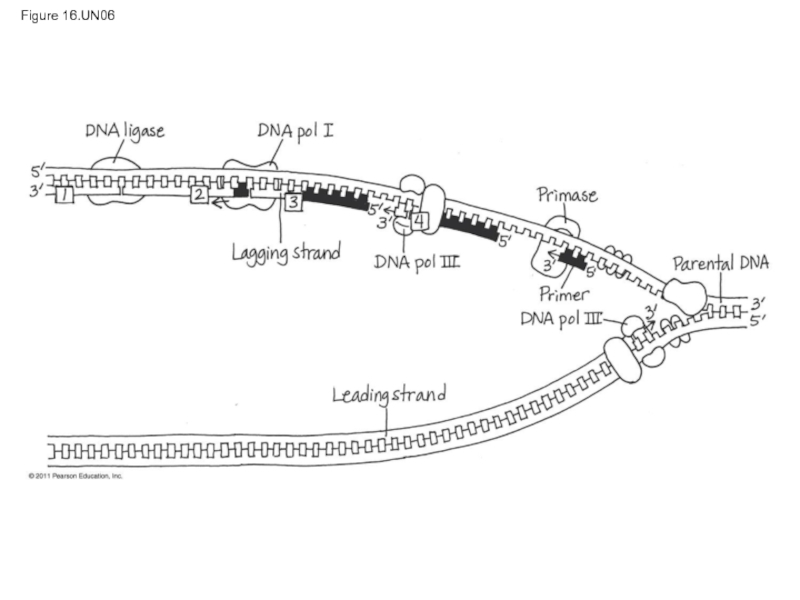
- 70. To elongate the other new strand, called
- 71. Animation: Lagging Strand Right-click slide / select “Play”
- 72. Origin of replication Overview Leading strand Leading
- 73. Figure 16.16a Origin of replication Overview Leading
- 74. Figure 16.16b-1 Template strand 3′ 3′ 5′ 5′
- 75. Figure 16.16b-2 Template strand RNA primer for
- 76. Figure 16.16b-3 Template strand RNA primer for
- 77. Figure 16.16b-4 Template strand RNA primer for
- 78. Figure 16.16b-5 Template strand RNA primer for
- 79. Figure 16.16b-6 Template strand RNA primer for
- 80. Figure 16.17 Overview Leading strand Origin of
- 81. Figure 16.17a Overview Leading strand Origin of
- 82. Overview Leading strand Origin of replication
- 83. The DNA Replication Complex The proteins that
- 84. Animation: DNA Replication Review Right-click slide / select “Play”
- 85. Figure 16.18 Parental DNA DNA pol III
- 86. Proofreading and Repairing DNA DNA polymerases proofread
- 87. Figure 16.19 Nuclease DNA polymerase DNA
- 88. Evolutionary Significance of Altered DNA Nucleotides Error
- 89. Replicating the Ends of DNA Molecules Limitations
- 90. Figure 16.20 Ends of parental DNA strands
- 91. Figure 16.20a Ends of parental DNA strands
- 92. Figure 16.20b Second round of replication Further
- 93. Eukaryotic chromosomal DNA molecules have special nucleotide
- 94. Figure 16.21 1 μm
- 95. If chromosomes of germ cells became shorter
- 96. The shortening of telomeres might protect cells
- 97. Concept 16.3 A chromosome consists of a
- 98. Chromatin, a complex of DNA and protein,
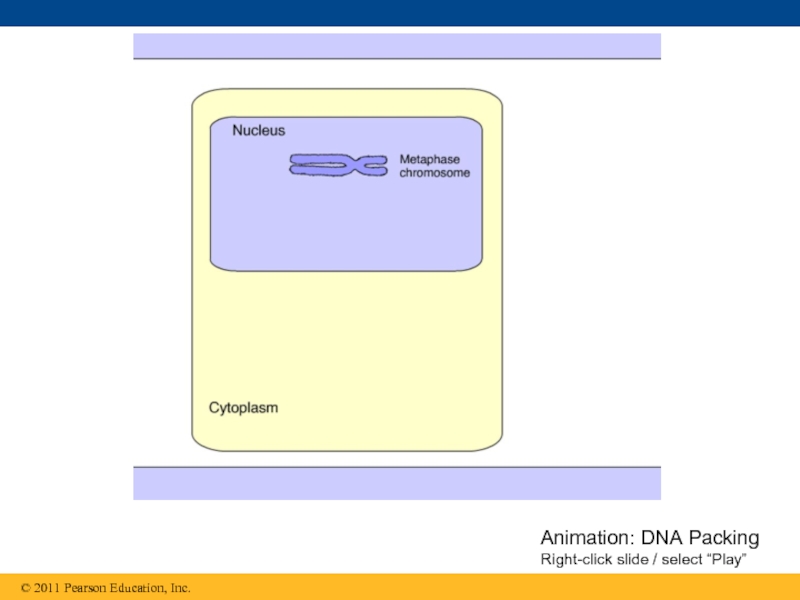
- 99. Animation: DNA Packing Right-click slide / select “Play”
- 100. Figure 16.22a DNA double helix (2 nm
- 101. Figure 16.22b 30-nm fiber 30-nm fiber Loops
- 102. Figure 16.22c DNA double helix (2 nm in diameter)
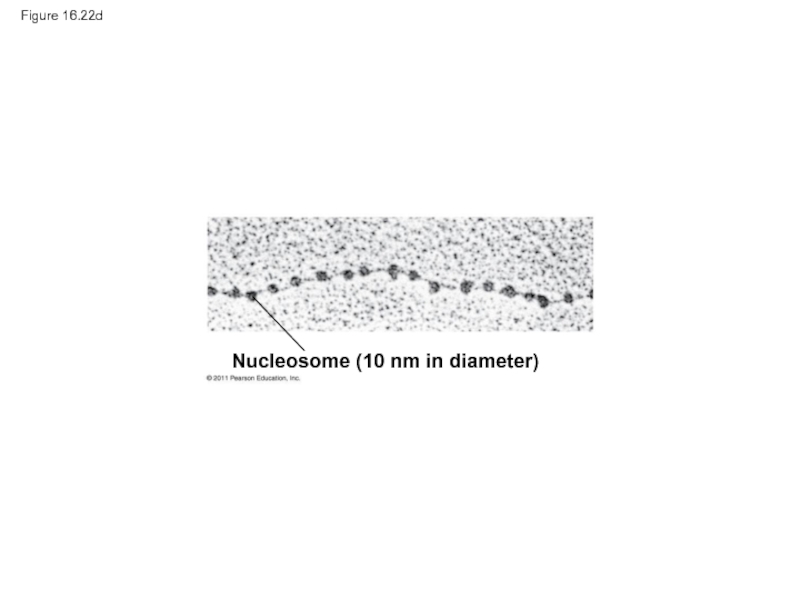
- 103. Figure 16.22d Nucleosome (10 nm in diameter)
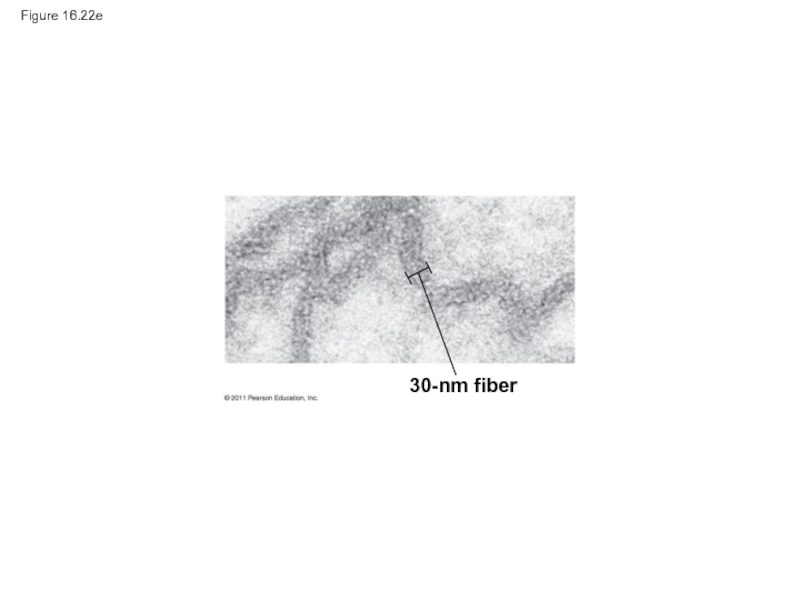
- 104. Figure 16.22e 30-nm fiber
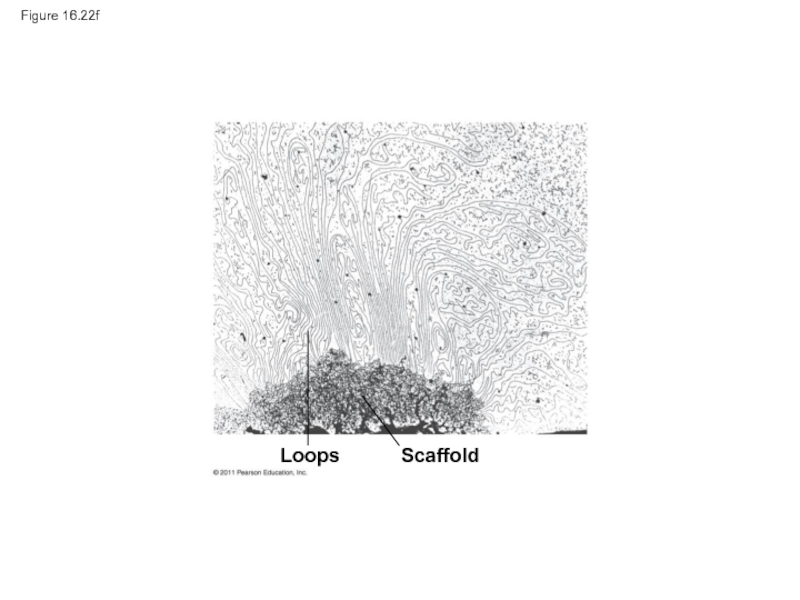
- 105. Figure 16.22f Loops Scaffold
- 106. Figure 16.22g Chromatid (700 nm)
- 107. Chromatin undergoes changes in packing during the
- 108. Figure 16.23 5 μm
- 109. Figure 16.23a
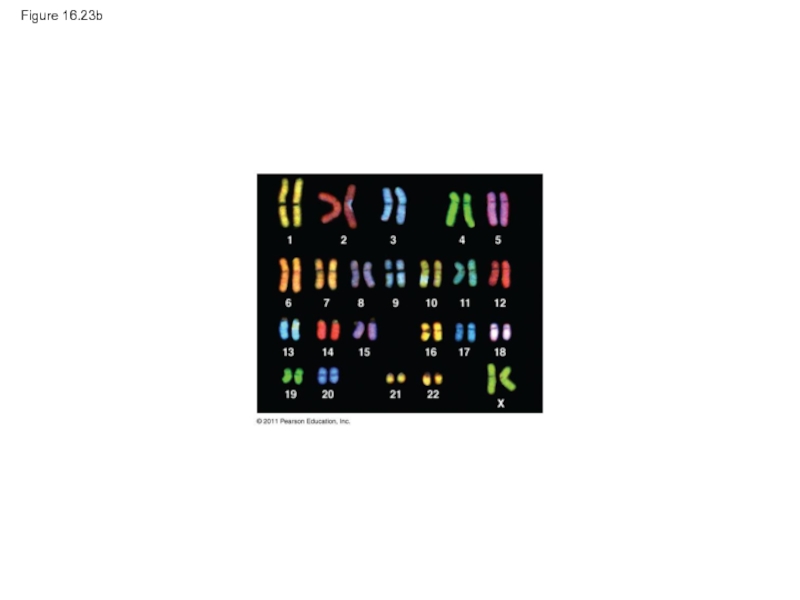
- 110. Figure 16.23b
- 111. Figure 16.23c 5 μm
- 112. Most chromatin is loosely packed in the
- 113. Histones can undergo chemical modifications that result in changes in chromatin organization
- 114. Figure 16.UN02 Sugar-phosphate backbone Nitrogenous bases Hydrogen
- 115. Figure 16.UN03 DNA pol III synthesizes leading
- 116. Figure 16.UN04
- 117. Figure 16.UN05
- 118. Figure 16.UN06
- 119. Figure 16.UN07
Слайд 2Overview: Life’s Operating Instructions
In 1953, James Watson and Francis Crick introduced
DNA, the substance of inheritance, is the most celebrated molecule of our time
Hereditary information is encoded in DNA and reproduced in all cells of the body
This DNA program directs the development of biochemical, anatomical, physiological, and (to some extent) behavioral traits
Слайд 4Concept 16.1: DNA is the genetic material
Early in the 20th century,
Слайд 5The Search for the Genetic Material: Scientific Inquiry
When T. H. Morgan’s
The key factor in determining the genetic material was choosing appropriate experimental organisms
The role of DNA in heredity was first discovered by studying bacteria and the viruses that infect them
Слайд 6Evidence That DNA Can Transform Bacteria
The discovery of the genetic role
Griffith worked with two strains of a bacterium, one pathogenic and one harmless
Слайд 7
When he mixed heat-killed remains of the pathogenic strain with living
He called this phenomenon transformation, now defined as a change in genotype and phenotype due to assimilation of foreign DNA
Слайд 8Living S cells
(control)
Living R cells
(control)
Heat-killed
S cells
(control)
Mixture of
heat-killed
S cells and
living R cells
Mouse
Mouse dies
Mouse healthy
Mouse healthy
Living S cells
EXPERIMENT
RESULTS
Figure 16.2
Слайд 9In 1944, Oswald Avery, Maclyn McCarty, and Colin MacLeod announced that
Their conclusion was based on experimental evidence that only DNA worked in transforming harmless bacteria into pathogenic bacteria
Many biologists remained skeptical, mainly because little was known about DNA
Слайд 10Evidence That Viral DNA Can Program Cells
More evidence for DNA as
Such viruses, called bacteriophages (or phages), are widely used in molecular genetics research
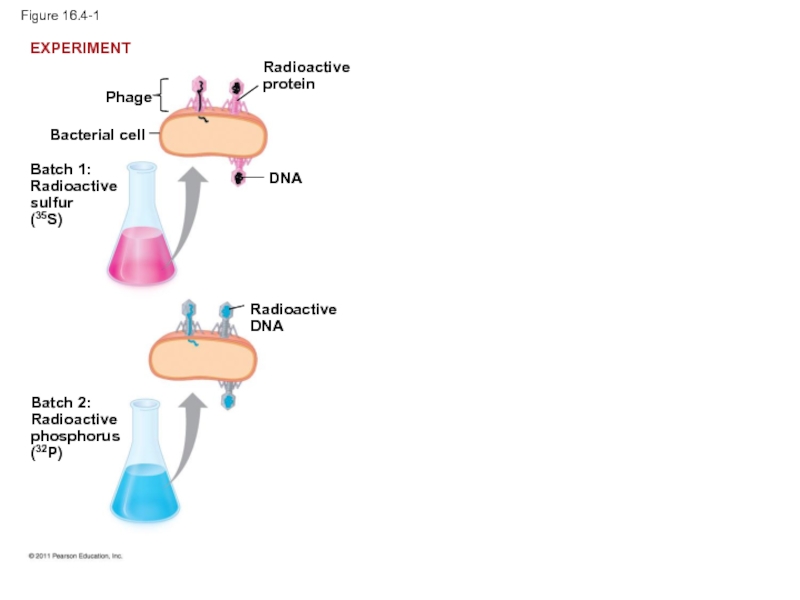
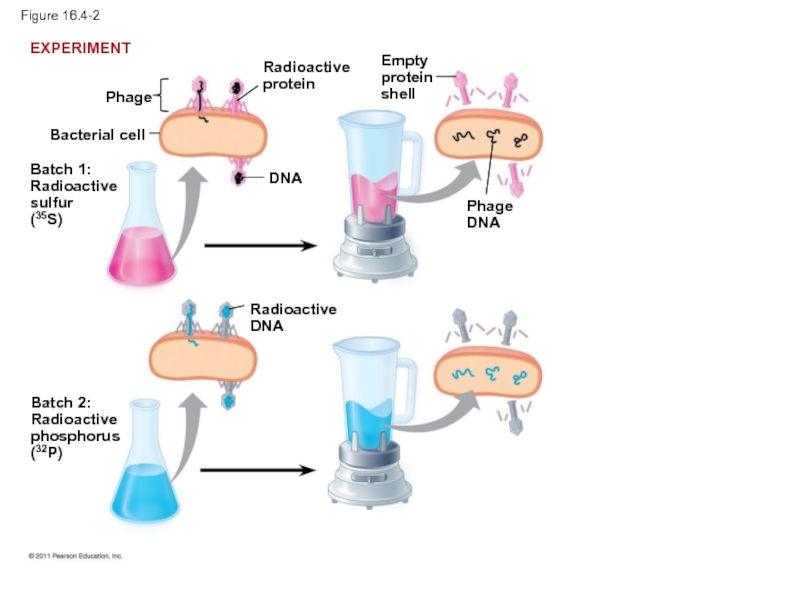
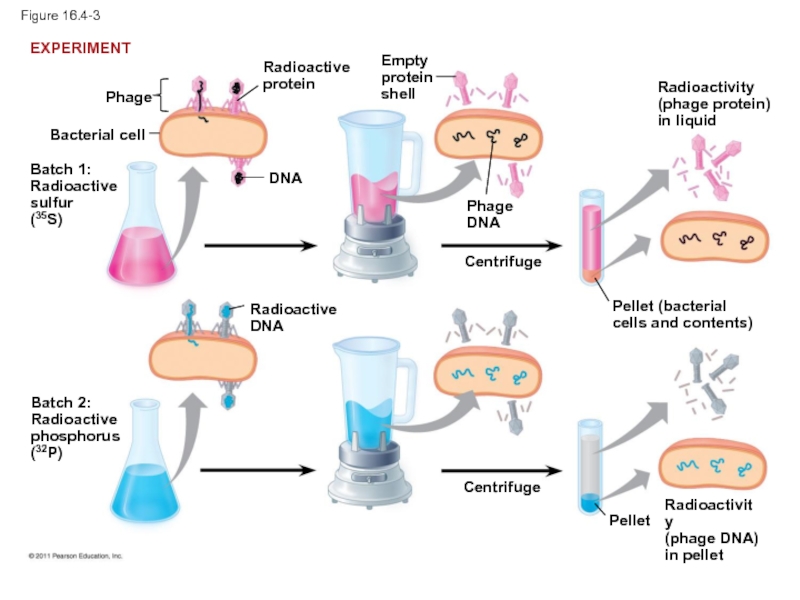
Слайд 13In 1952, Alfred Hershey and Martha Chase performed experiments showing that
To determine this, they designed an experiment showing that only one of the two components of T2 (DNA or protein) enters an E. coli cell during infection
They concluded that the injected DNA of the phage provides the genetic information
Слайд 15Figure 16.4-1
Bacterial cell
Phage
Batch 1:
Radioactive
sulfur
(35S)
DNA
Batch 2:
Radioactive
phosphorus
(32P)
Radioactive
DNA
EXPERIMENT
Radioactive
protein
Слайд 16Figure 16.4-2
Bacterial cell
Phage
Batch 1:
Radioactive
sulfur
(35S)
Radioactive
protein
DNA
Batch 2:
Radioactive
phosphorus
(32P)
Radioactive
DNA
Empty
protein
shell
Phage
DNA
EXPERIMENT
Слайд 17Figure 16.4-3
Bacterial cell
Phage
Batch 1:
Radioactive
sulfur
(35S)
Radioactive
protein
DNA
Batch 2:
Radioactive
phosphorus
(32P)
Radioactive
DNA
Empty
protein
shell
Phage
DNA
Centrifuge
Centrifuge
Radioactivity
(phage protein)
in liquid
Pellet (bacterial
cells and contents)
Pellet
Radioactivity
(phage DNA)
in
EXPERIMENT
Слайд 18Additional Evidence That DNA Is the Genetic Material
It was known that
In 1950, Erwin Chargaff reported that DNA composition varies from one species to the next
This evidence of diversity made DNA a more credible candidate for the genetic material
Слайд 20Two findings became known as Chargaff’s rules
The base composition of DNA
In any species the number of A and T bases are equal and the number of G and C bases are equal
The basis for these rules was not understood until the discovery of the double helix
Слайд 21Figure 16.5
Sugar–phosphate
backbone
Nitrogenous bases
Thymine (T)
Adenine (A)
Cytosine (C)
Guanine (G)
Nitrogenous base
Phosphate
DNA
nucleotide
Sugar
(deoxyribose)
3′ end
5′ end
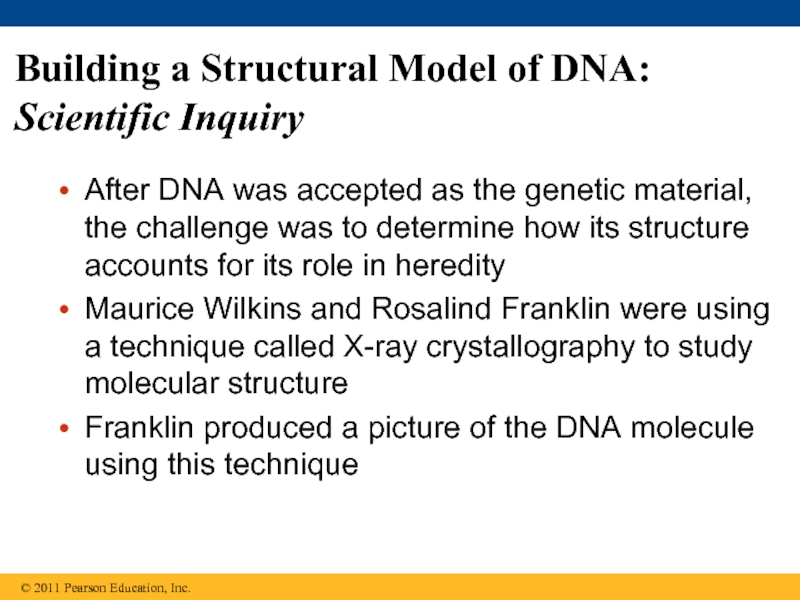
Слайд 22Building a Structural Model of DNA: Scientific Inquiry
After DNA was accepted
Maurice Wilkins and Rosalind Franklin were using a technique called X-ray crystallography to study molecular structure
Franklin produced a picture of the DNA molecule using this technique
Слайд 26Franklin’s X-ray crystallographic images of DNA enabled Watson to deduce that
The X-ray images also enabled Watson to deduce the width of the helix and the spacing of the nitrogenous bases
The pattern in the photo suggested that the DNA molecule was made up of two strands, forming a double helix
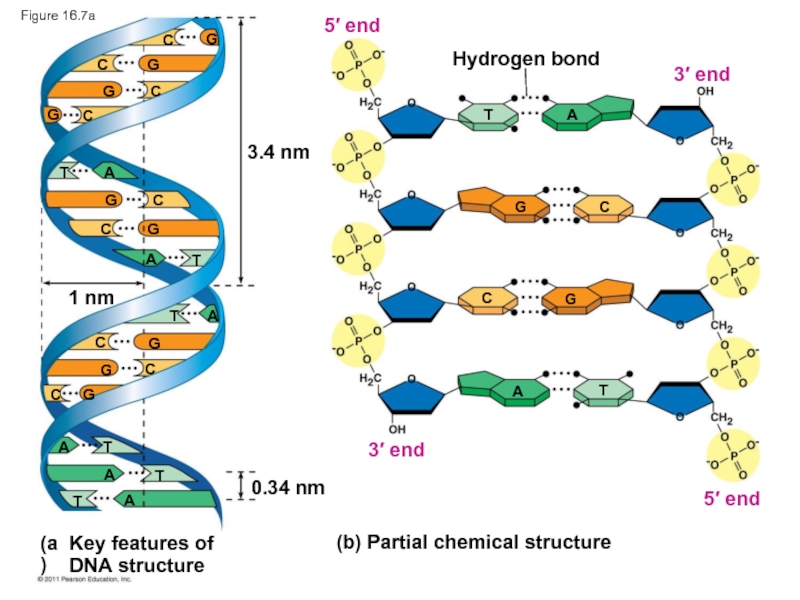
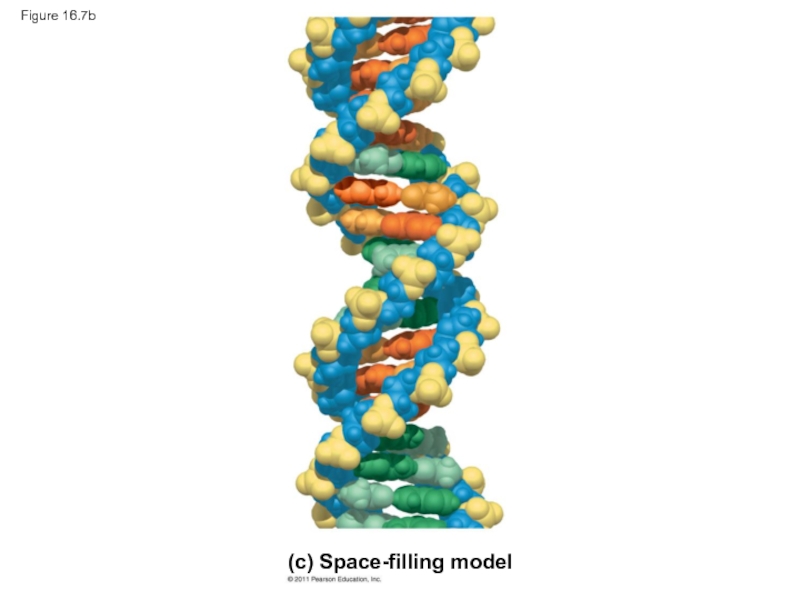
Слайд 28Figure 16.7
3.4 nm
1 nm
0.34 nm
Hydrogen bond
(b) Partial chemical structure
3′ end
5′ end
3′
5′ end
T
T
A
A
G
G
C
C
C
C
C
C
C
C
C
C
C
G
G
G
G
G
G
G
G
G
T
T
T
T
T
T
A
A
A
A
A
A
Слайд 293.4 nm
1 nm
0.34 nm
Hydrogen bond
(b) Partial chemical structure
3′ end
5′ end
3′ end
5′
T
T
A
A
G
G
C
C
C
C
C
C
C
C
C
C
C
G
G
G
G
G
G
G
G
G
T
T
T
T
T
T
A
A
A
A
A
A
Figure 16.7a
Слайд 31Watson and Crick built models of a double helix to conform
Franklin had concluded that there were two outer sugar-phosphate backbones, with the nitrogenous bases paired in the molecule’s interior
Watson built a model in which the backbones were antiparallel (their subunits run in opposite directions)
Слайд 32At first, Watson and Crick thought the bases paired like with
Instead, pairing a purine with a pyrimidine resulted in a uniform width consistent with the X-ray data
Слайд 33Figure 16.UN01
Purine + purine: too wide
Pyrimidine + pyrimidine: too narrow
Purine +
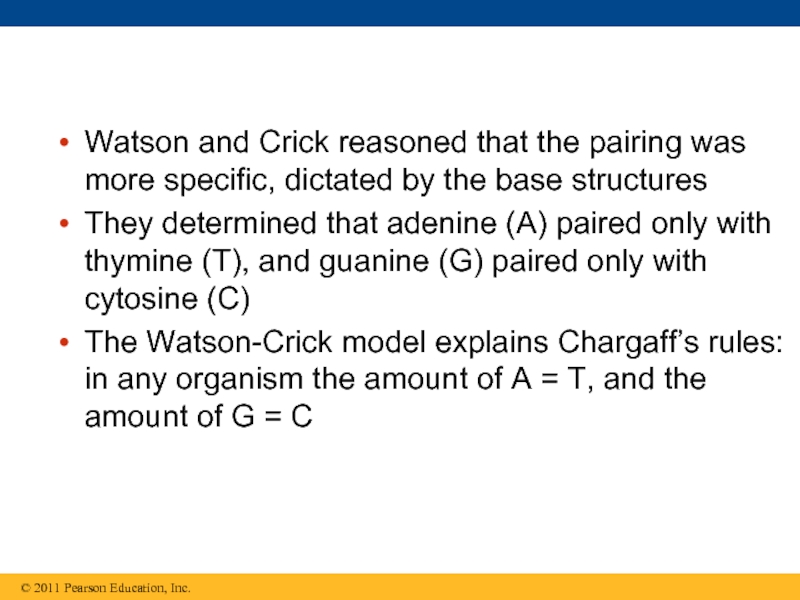
Слайд 34Watson and Crick reasoned that the pairing was more specific, dictated
They determined that adenine (A) paired only with thymine (T), and guanine (G) paired only with cytosine (C)
The Watson-Crick model explains Chargaff’s rules: in any organism the amount of A = T, and the amount of G = C
Слайд 36Concept 16.2: Many proteins work together in DNA replication and repair
The
Watson and Crick noted that the specific base pairing suggested a possible copying mechanism for genetic material
Слайд 37The Basic Principle: Base Pairing to a Template Strand
Since the two
In DNA replication, the parent molecule unwinds, and two new daughter strands are built based on base-pairing rules
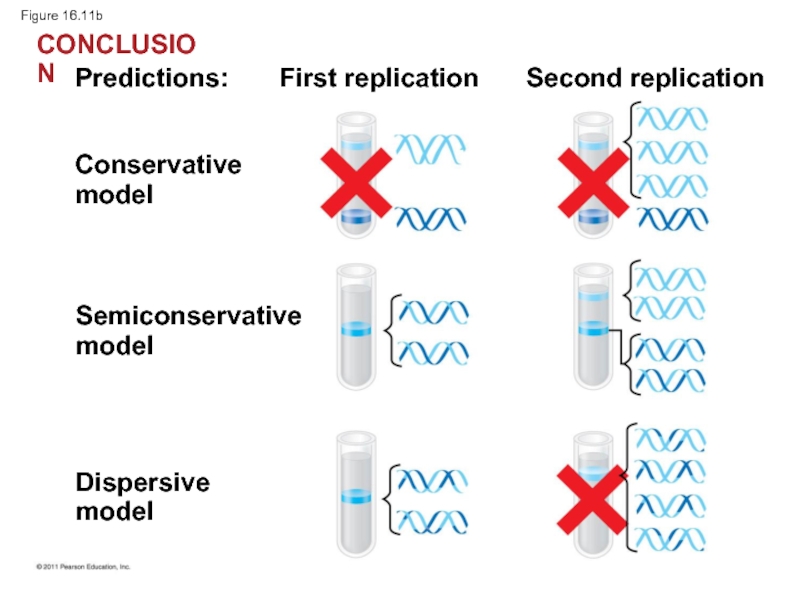
Слайд 42Watson and Crick’s semiconservative model of replication predicts that when a
Competing models were the conservative model (the two parent strands rejoin) and the dispersive model (each strand is a mix of old and new)
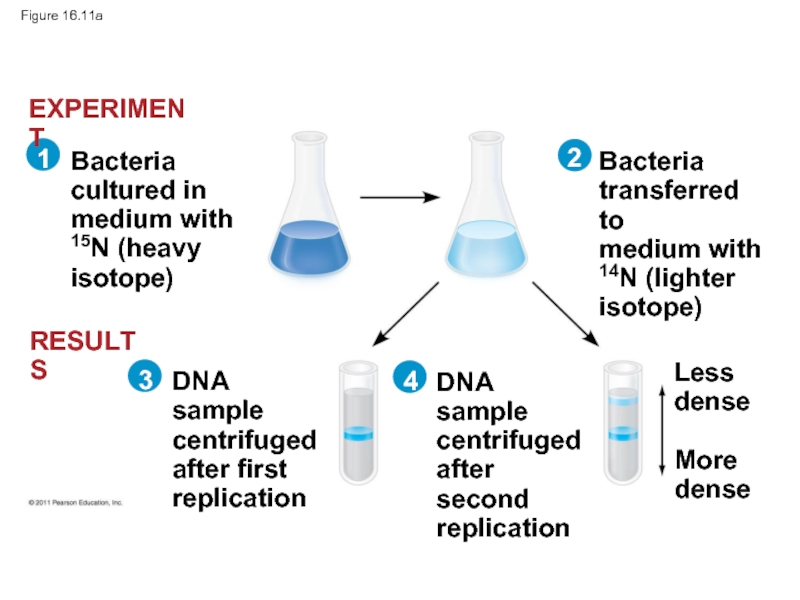
Слайд 44Experiments by Matthew Meselson and Franklin Stahl supported the semiconservative model
They labeled the nucleotides of the old strands with a heavy isotope of nitrogen, while any new nucleotides were labeled with a lighter isotope
Слайд 45The first replication produced a band of hybrid DNA, eliminating the
A second replication produced both light and hybrid DNA, eliminating the dispersive model and supporting the semiconservative model
Слайд 46Figure 16.11
Bacteria
cultured in
medium with
15N (heavy
isotope)
Bacteria
transferred to
medium with
14N (lighter
isotope)
DNA sample
centrifuged
after first
replication
DNA sample
centrifuged
after
Less
dense
More
dense
Predictions:
First replication
Second replication
Conservative
model
Semiconservative
model
Dispersive
model
EXPERIMENT
RESULTS
CONCLUSION
Слайд 47Figure 16.11a
Bacteria
cultured in
medium with
15N (heavy
isotope)
Bacteria
transferred to
medium with
14N (lighter
isotope)
DNA sample
centrifuged
after first
replication
DNA sample
centrifuged
after
Less
dense
More
dense
EXPERIMENT
RESULTS
Слайд 48Figure 16.11b
Predictions:
First replication
Second replication
Conservative
model
Semiconservative
model
Dispersive
model
CONCLUSION
Слайд 49DNA Replication: A Closer Look
The copying of DNA is remarkable in
More than a dozen enzymes and other proteins participate in DNA replication
Слайд 50Getting Started
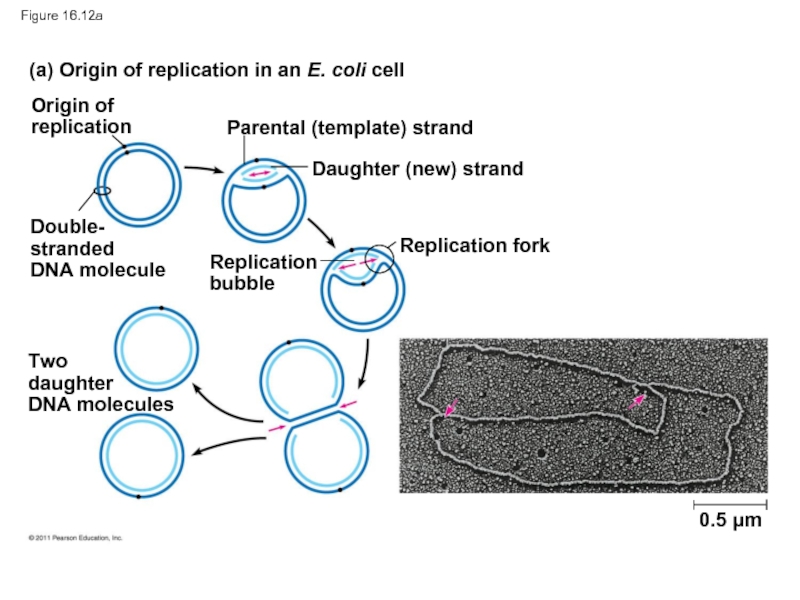
Replication begins at particular sites called origins of replication, where
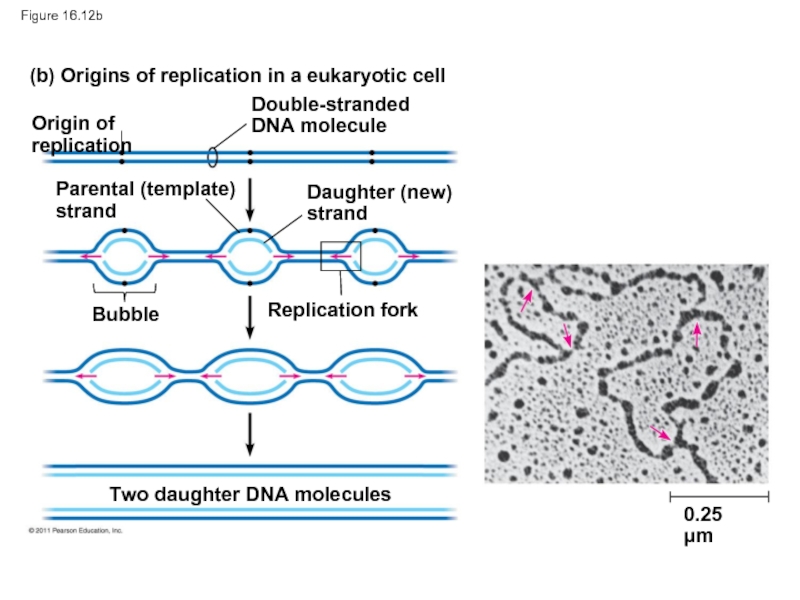
A eukaryotic chromosome may have hundreds or even thousands of origins of replication
Replication proceeds in both directions from each origin, until the entire molecule is copied
Слайд 52Figure 16.12
(a) Origin of replication in an E. coli cell
(b) Origins
Origin of
replication
Parental (template) strand
Double-
stranded
DNA molecule
Daughter (new)
strand
Replication
fork
Replication
bubble
Two daughter
DNA molecules
Origin of replication
Double-stranded
DNA molecule
Parental (template)
strand
Daughter (new)
strand
Bubble
Replication fork
Two daughter DNA molecules
0.5 μm
0.25 μm
Слайд 53Figure 16.12a
(a) Origin of replication in an E. coli cell
Origin of
replication
Parental
Double-
stranded
DNA molecule
Daughter (new) strand
Replication fork
Replication
bubble
Two
daughter
DNA molecules
0.5 μm
Слайд 54Figure 16.12b
(b) Origins of replication in a eukaryotic cell
Origin of replication
Double-stranded
DNA
Parental (template)
strand
Daughter (new)
strand
Bubble
Replication fork
Two daughter DNA molecules
0.25 μm
Слайд 57At the end of each replication bubble is a replication fork,
Helicases are enzymes that untwist the double helix at the replication forks
Single-strand binding proteins bind to and stabilize single-stranded DNA
Topoisomerase corrects “overwinding” ahead of replication forks by breaking, swiveling, and rejoining DNA strands
Слайд 58Figure 16.13
Topoisomerase
Primase
RNA
primer
Helicase
Single-strand binding
proteins
5′
3′
5′
5′
3′
3′
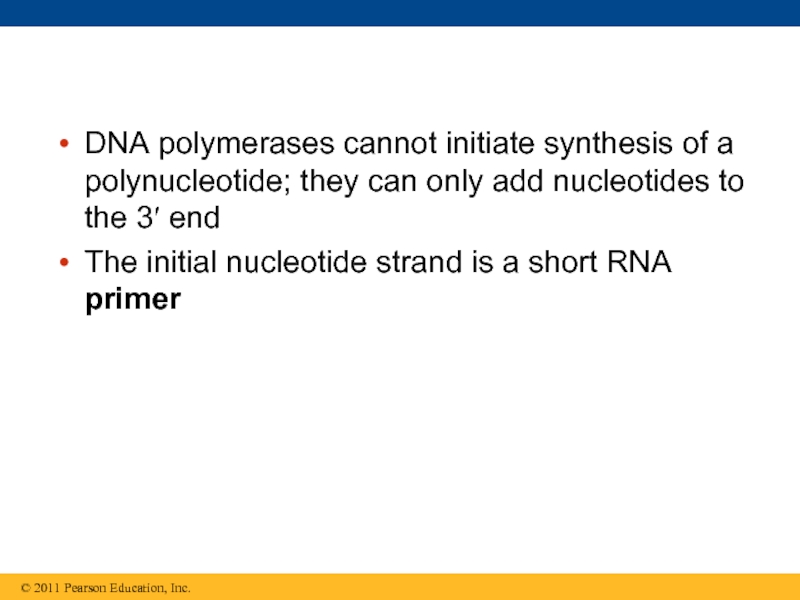
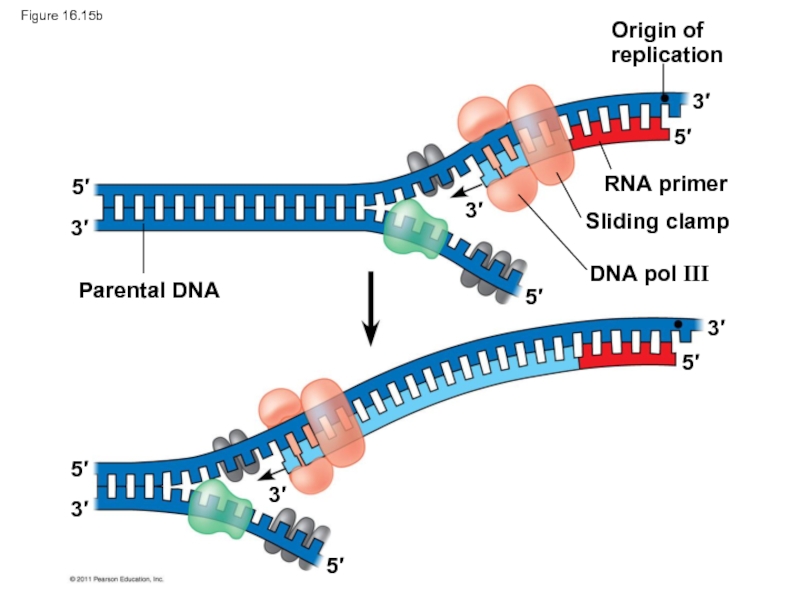
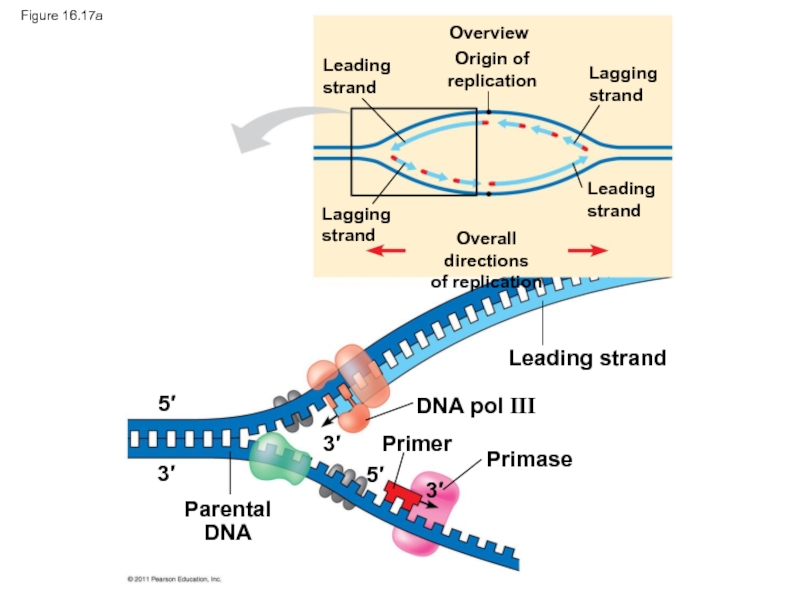
Слайд 59DNA polymerases cannot initiate synthesis of a polynucleotide; they can only
The initial nucleotide strand is a short RNA primer
Слайд 60An enzyme called primase can start an RNA chain from scratch
The primer is short (5–10 nucleotides long), and the 3′ end serves as the starting point for the new DNA strand
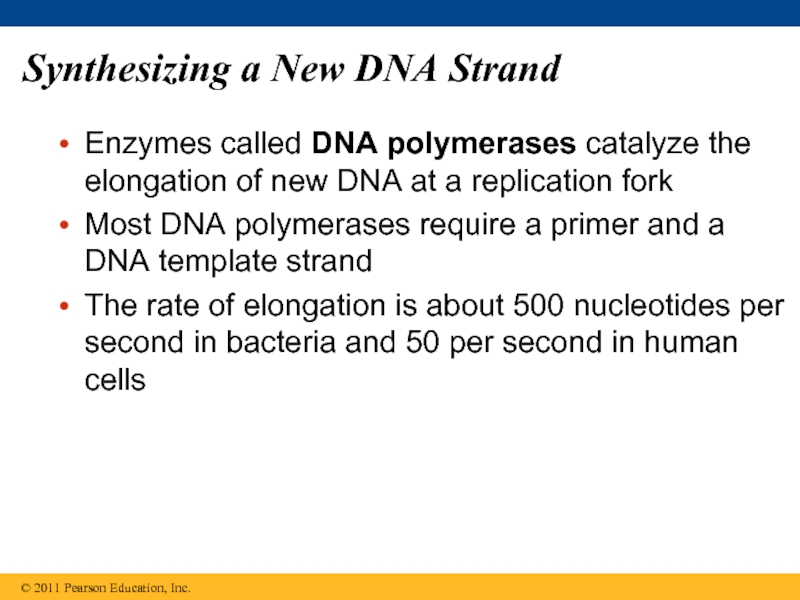
Слайд 61Synthesizing a New DNA Strand
Enzymes called DNA polymerases catalyze the elongation
Most DNA polymerases require a primer and a DNA template strand
The rate of elongation is about 500 nucleotides per second in bacteria and 50 per second in human cells
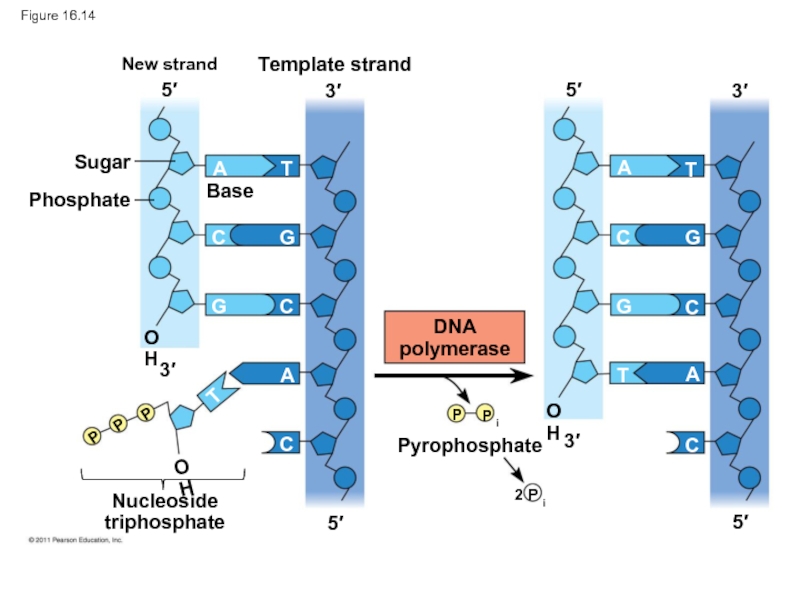
Слайд 62Each nucleotide that is added to a growing DNA strand is
dATP supplies adenine to DNA and is similar to the ATP of energy metabolism
The difference is in their sugars: dATP has deoxyribose while ATP has ribose
As each monomer of dATP joins the DNA strand, it loses two phosphate groups as a molecule of pyrophosphate
Слайд 63Figure 16.14
New strand
Template strand
Sugar
Phosphate
Base
Nucleoside
triphosphate
DNA
polymerase
Pyrophosphate
5′
5′
5′
5′
3′
3′
3′
3′
OH
OH
OH
P
P i
2 P i
P
P
P
A
A
A
A
T
T
T
T
C
C
C
C
C
C
G
G
G
G
Слайд 64Antiparallel Elongation
The antiparallel structure of the double helix affects replication
DNA polymerases
Слайд 65Along one template strand of DNA, the DNA polymerase synthesizes a
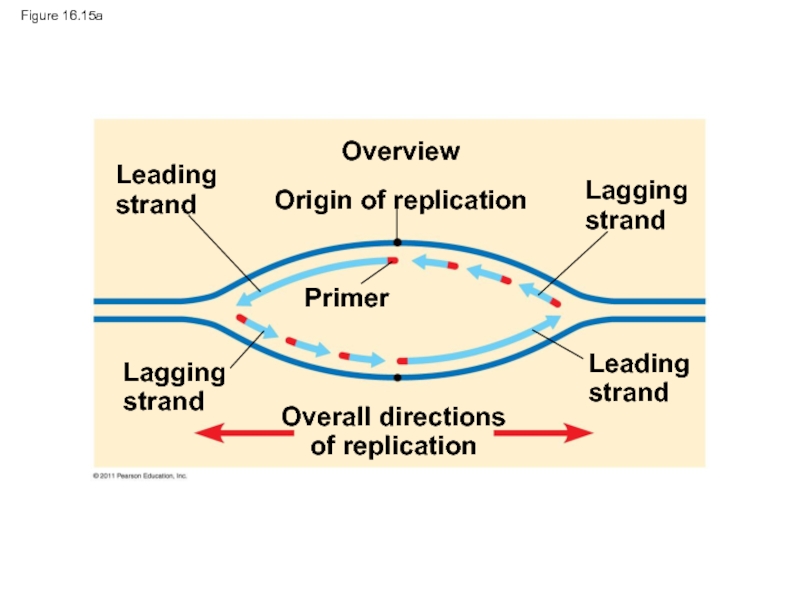
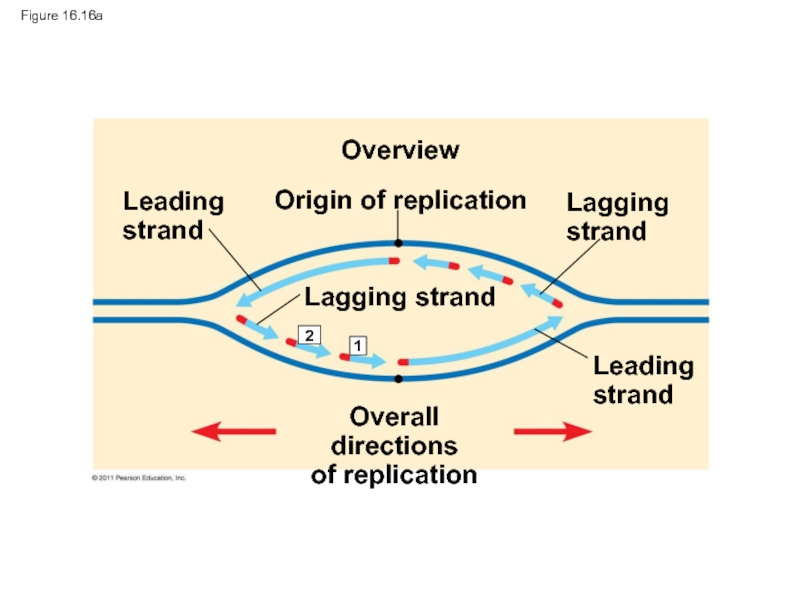
Слайд 67Figure 16.15
Leading
strand
Lagging
strand
Overview
Origin of replication
Lagging
strand
Leading
strand
Primer
Overall directions
of replication
Origin of
replication
RNA primer
Sliding clamp
DNA pol
Parental DNA
3′
5′
5′
3′
3′
5′
3′
5′
3′
5′
3′
5′
Слайд 68Figure 16.15a
Leading
strand
Lagging
strand
Overview
Origin of replication
Lagging
strand
Leading
strand
Primer
Overall directions
of replication
Слайд 69Origin of
replication
RNA primer
Sliding clamp
DNA pol III
Parental DNA
3′
5′
5′
3′
3′
5′
3′
5′
3′
5′
3′
5′
Figure 16.15b
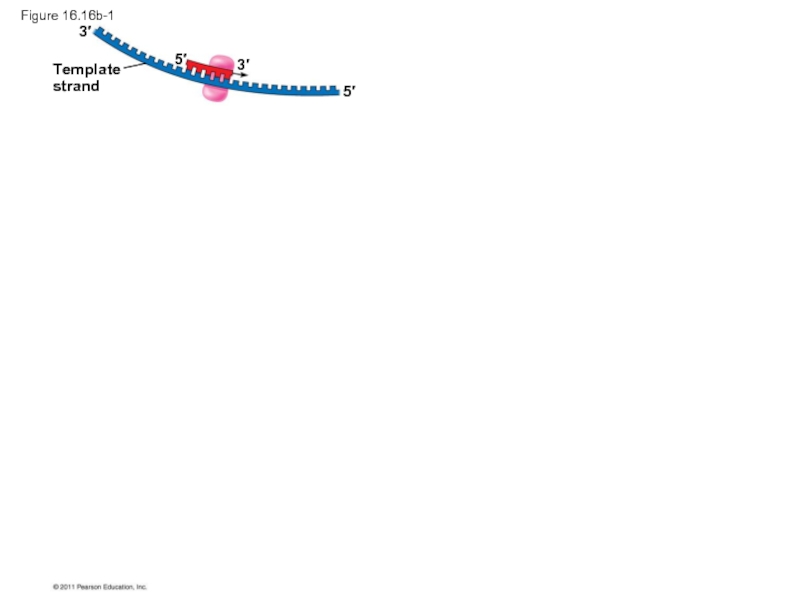
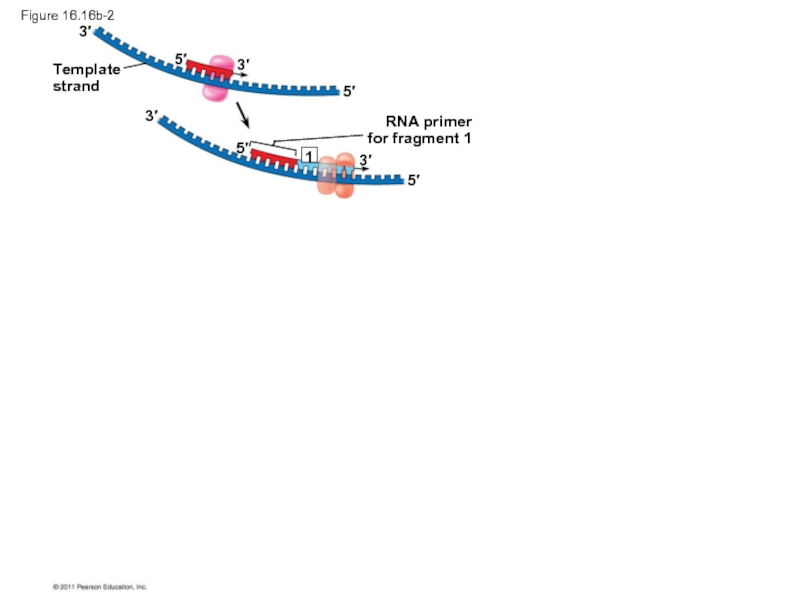
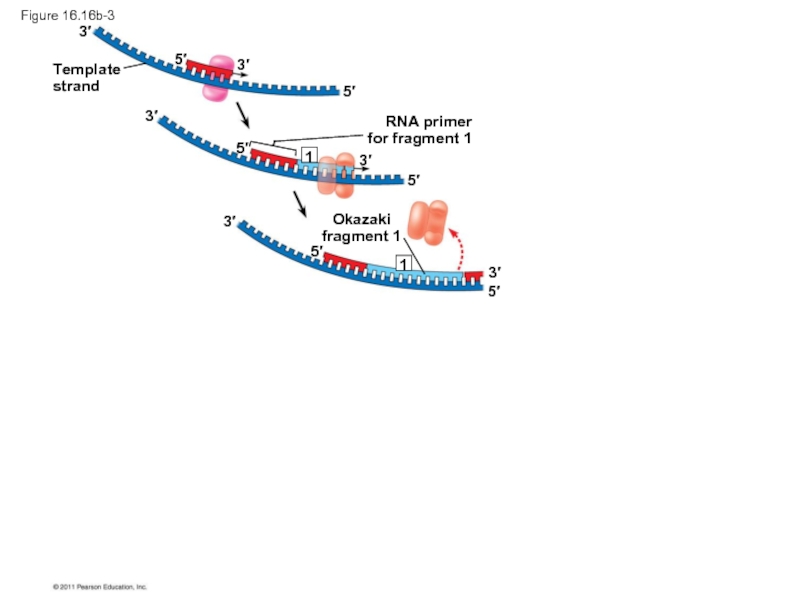
Слайд 70To elongate the other new strand, called the lagging strand, DNA
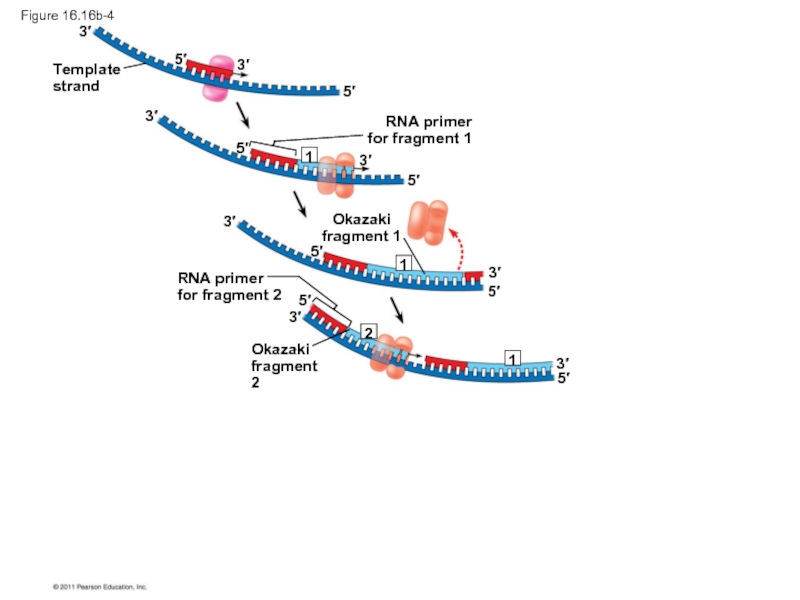
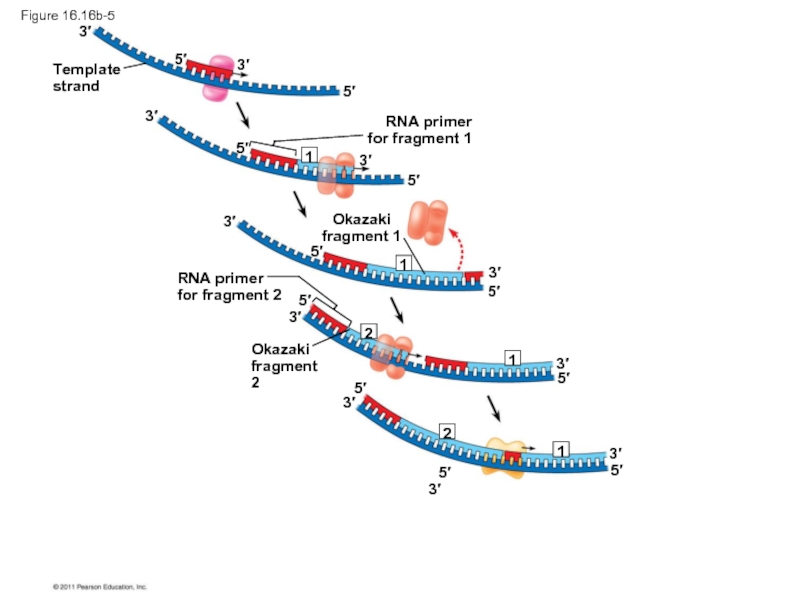
The lagging strand is synthesized as a series of segments called Okazaki fragments, which are joined together by DNA ligase
Слайд 72Origin of replication
Overview
Leading
strand
Leading
strand
Lagging
strand
Lagging strand
Overall directions
of replication
Template
strand
RNA primer
for fragment 1
Okazaki
fragment 1
RNA primer
for
Okazaki
fragment 2
Overall direction of replication
3′
3′
3′
3′
3′
3′
3′
3′
3′
3′
3′
3′
5′
5′
5′
5′
5′
5′
5′
5′
5′
5′
5′
5′
2
2
2
1
1
1
1
1
2
1
Figure 16.16
Слайд 73Figure 16.16a
Origin of replication
Overview
Leading
strand
Leading
strand
Lagging
strand
Lagging strand
Overall directions
of replication
1
2
Слайд 76Figure 16.16b-3
Template
strand
RNA primer
for fragment 1
Okazaki
fragment 1
3′
3′
3′
3′
3′
3′
5′
5′
5′
5′
5′
5′
1
1
Слайд 77Figure 16.16b-4
Template
strand
RNA primer
for fragment 1
Okazaki
fragment 1
RNA primer
for fragment 2
Okazaki
fragment 2
3′
3′
3′
3′
3′
3′
3′
3′
5′
5′
5′
5′
5′
5′
5′
5′
2
1
1
1
Слайд 78Figure 16.16b-5
Template
strand
RNA primer
for fragment 1
Okazaki
fragment 1
RNA primer
for fragment 2
Okazaki
fragment 2
3′
3′
3′
3′
3′
3′
3′
3′
3′
3′
3′
5′
5′
5′
5′
5′
5′
5′
5′
5′
5′
5′
2
2
1
1
1
1
Слайд 79Figure 16.16b-6
Template
strand
RNA primer
for fragment 1
Okazaki
fragment 1
RNA primer
for fragment 2
Okazaki
fragment 2
Overall direction
3′
3′
3′
3′
3′
3′
3′
3′
3′
3′
3′
3′
5′
5′
5′
5′
5′
5′
5′
5′
5′
5′
5′
5′
2
2
2
1
1
1
1
1
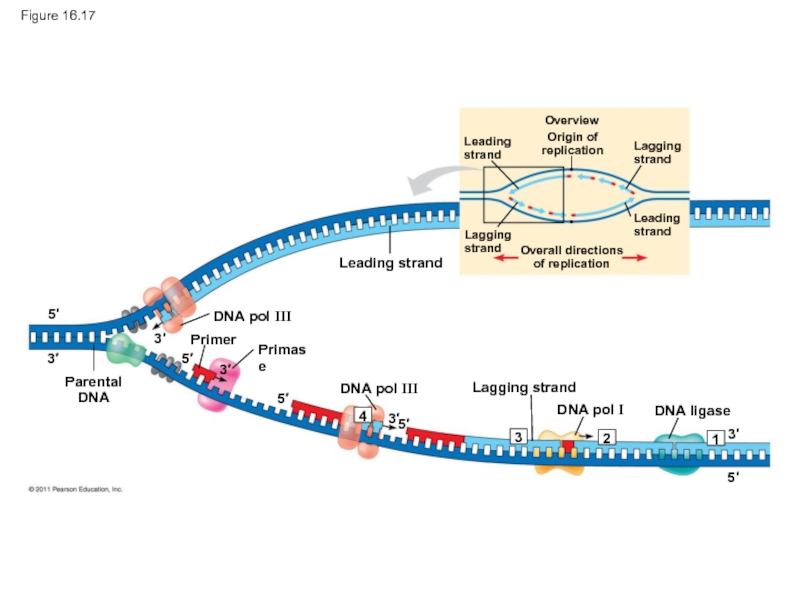
Слайд 80Figure 16.17
Overview
Leading
strand
Origin of
replication
Lagging
strand
Leading
strand
Lagging
strand
Overall directions
of replication
Leading strand
DNA pol III
DNA pol III
Lagging
DNA pol I
DNA ligase
Primer
Primase
Parental
DNA
5′
5′
5′
5′
5′
3′
3′
3′
3′
3′
3
2
1
4
Слайд 81Figure 16.17a
Overview
Leading
strand
Origin of
replication
Lagging
strand
Leading
strand
Lagging
strand
Overall directions
of replication
Leading strand
DNA pol III
Primer
Primase
Parental
DNA
5′
5′
3′
3′
3′
Слайд 82Overview
Leading
strand
Origin of
replication
Lagging
strand
Leading
strand
Lagging
strand
Overall directions
of replication
Leading strand
Primer
DNA pol III
DNA pol I
Lagging strand
DNA
5′
5′
5′
3′
3′
3′
3
4
2
1
Figure 16.17b
Слайд 83The DNA Replication Complex
The proteins that participate in DNA replication form
The DNA replication machine may be stationary during the replication process
Recent studies support a model in which DNA polymerase molecules “reel in” parental DNA and “extrude” newly made daughter DNA molecules
Слайд 85Figure 16.18
Parental DNA
DNA pol III
Leading strand
Connecting
protein
Helicase
Lagging strand
DNA
pol III
Lagging
strand
template
5′
5′
5′
5′
5′
5′
3′
3′
3′
3′
3′
3′
Слайд 86Proofreading and Repairing DNA
DNA polymerases proofread newly made DNA, replacing any
In mismatch repair of DNA, repair enzymes correct errors in base pairing
DNA can be damaged by exposure to harmful chemical or physical agents such as cigarette smoke and X-rays; it can also undergo spontaneous changes
In nucleotide excision repair, a nuclease cuts out and replaces damaged stretches of DNA
Слайд 88Evolutionary Significance of Altered DNA Nucleotides
Error rate after proofreading repair is
Sequence changes may become permanent and can be passed on to the next generation
These changes (mutations) are the source of the genetic variation upon which natural selection operates
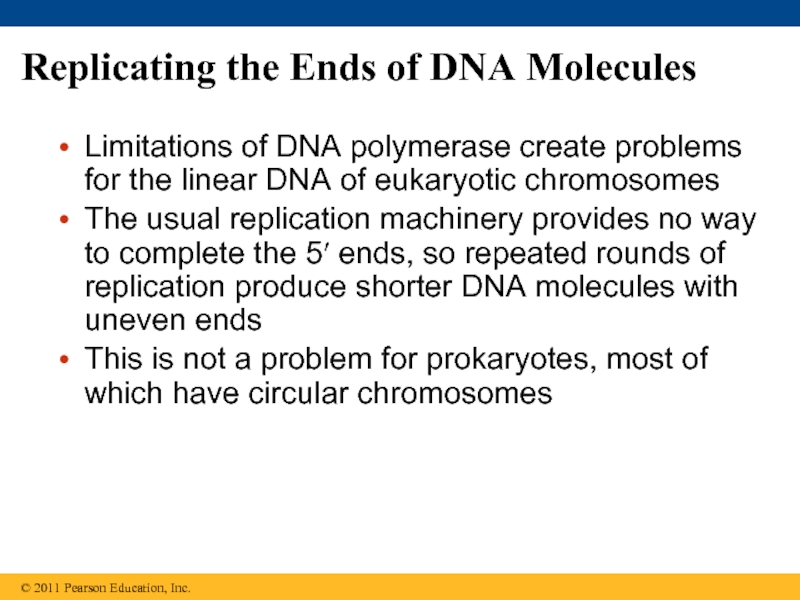
Слайд 89Replicating the Ends of DNA Molecules
Limitations of DNA polymerase create problems
The usual replication machinery provides no way to complete the 5′ ends, so repeated rounds of replication produce shorter DNA molecules with uneven ends
This is not a problem for prokaryotes, most of which have circular chromosomes
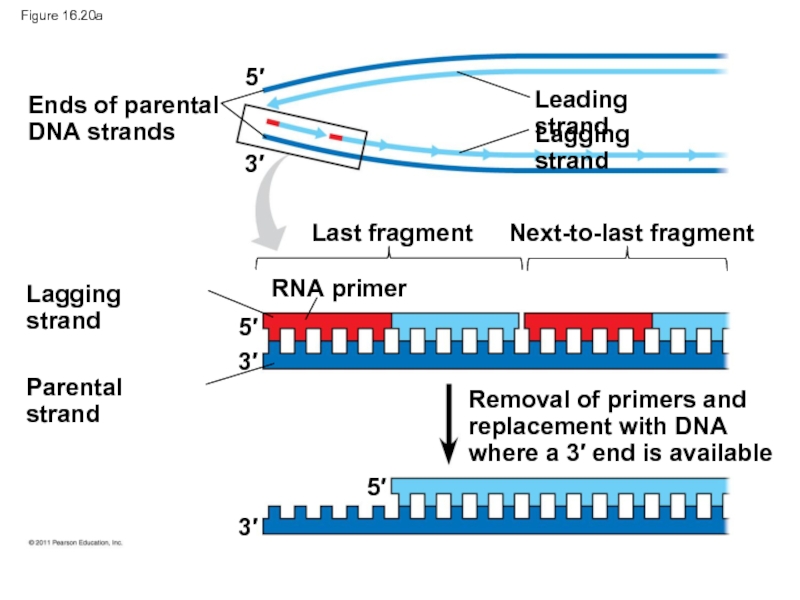
Слайд 90Figure 16.20
Ends of parental
DNA strands
Leading strand
Lagging strand
Last fragment
Next-to-last fragment
Lagging strand
RNA primer
Parental
Removal of primers and
replacement with DNA
where a 3′ end is available
Second round
of replication
Further rounds
of replication
New leading strand
New lagging strand
Shorter and shorter daughter molecules
3′
3′
3′
3′
3′
5′
5′
5′
5′
5′
Слайд 91Figure 16.20a
Ends of parental
DNA strands
Leading strand
Lagging strand
Last fragment
Next-to-last fragment
Lagging strand
RNA primer
Parental
Removal of primers and
replacement with DNA
where a 3′ end is available
3′
3′
3′
5′
5′
5′
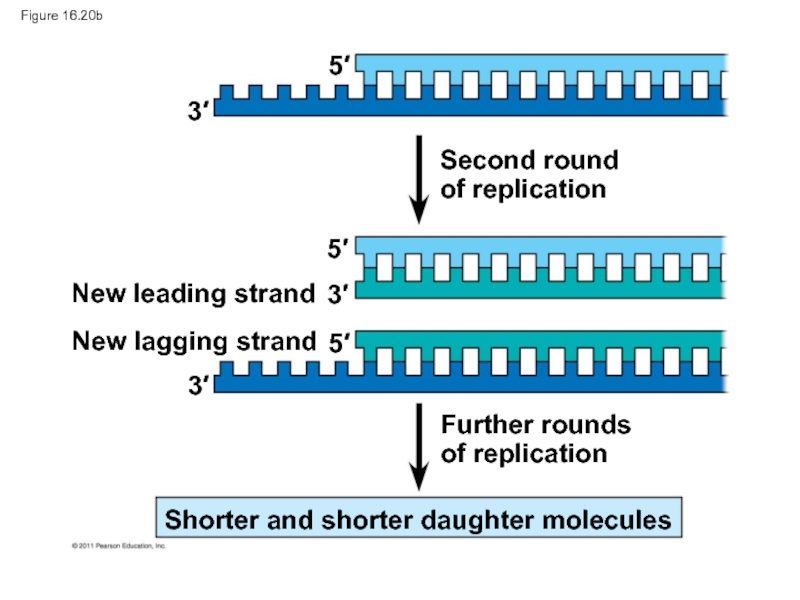
Слайд 92Figure 16.20b
Second round
of replication
Further rounds
of replication
New leading strand
New lagging strand
Shorter and
3′
3′
3′
5′
5′
5′
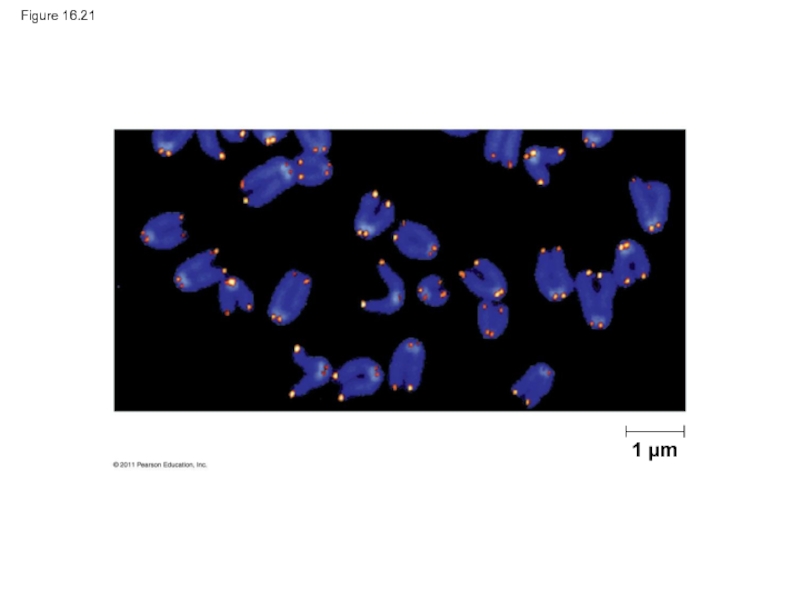
Слайд 93Eukaryotic chromosomal DNA molecules have special nucleotide sequences at their ends
Telomeres do not prevent the shortening of DNA molecules, but they do postpone the erosion of genes near the ends of DNA molecules
It has been proposed that the shortening of telomeres is connected to aging
Слайд 95If chromosomes of germ cells became shorter in every cell cycle,
An enzyme called telomerase catalyzes the lengthening of telomeres in germ cells
Слайд 96The shortening of telomeres might protect cells from cancerous growth by
There is evidence of telomerase activity in cancer cells, which may allow cancer cells to persist
Слайд 97Concept 16.3 A chromosome consists of a DNA molecule packed together
The bacterial chromosome is a double-stranded, circular DNA molecule associated with a small amount of protein
Eukaryotic chromosomes have linear DNA molecules associated with a large amount of protein
In a bacterium, the DNA is “supercoiled” and found in a region of the cell called the nucleoid
Слайд 98Chromatin, a complex of DNA and protein, is found in the
Chromosomes fit into the nucleus through an elaborate, multilevel system of packing
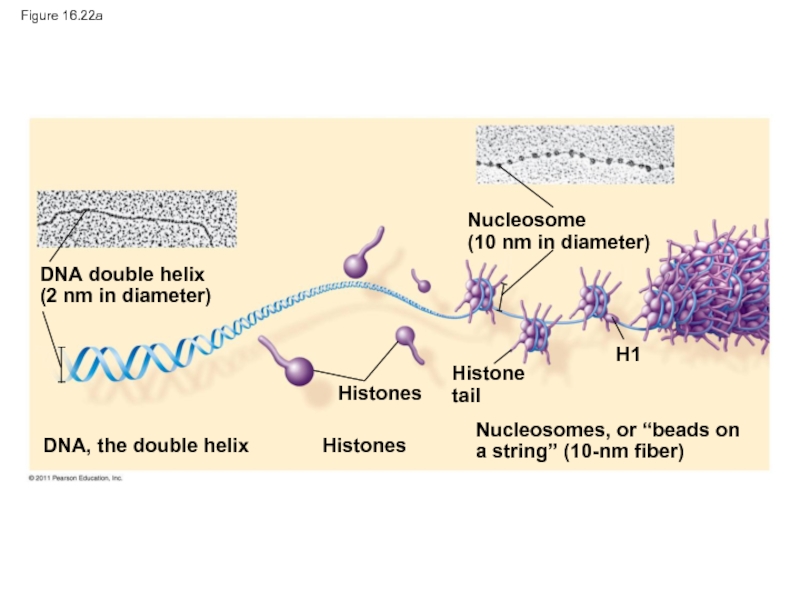
Слайд 100Figure 16.22a
DNA double helix
(2 nm in diameter)
DNA, the double helix
Nucleosome
(10 nm
Histones
Histones
Histone
tail
H1
Nucleosomes, or “beads on
a string” (10-nm fiber)
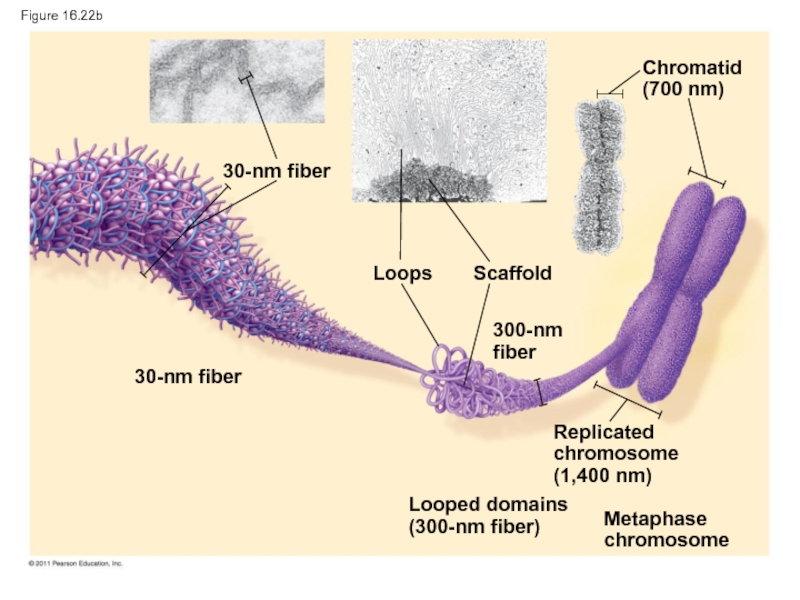
Слайд 101Figure 16.22b
30-nm fiber
30-nm fiber
Loops
Scaffold
300-nm fiber
Chromatid
(700 nm)
Replicated
chromosome
(1,400 nm)
Looped domains
(300-nm fiber)
Metaphase
chromosome
Слайд 107Chromatin undergoes changes in packing during the cell cycle
At interphase, some
Though interphase chromosomes are not highly condensed, they still occupy specific restricted regions in the nucleus
Слайд 112Most chromatin is loosely packed in the nucleus during interphase and
Loosely packed chromatin is called euchromatin
During interphase a few regions of chromatin (centromeres and telomeres) are highly condensed into heterochromatin
Dense packing of the heterochromatin makes it difficult for the cell to express genetic information coded in these regions
Слайд 113Histones can undergo chemical modifications that result in changes in chromatin
Слайд 115Figure 16.UN03
DNA pol III synthesizes
leading strand continuously
Parental
DNA
DNA pol III starts DNA
synthesis
Origin of
replication
Helicase
Primase synthesizes
a short RNA primer
DNA pol I replaces the RNA
primer with DNA nucleotides
3′
3′
3′
5′
5′
5′
5′
Lagging strand synthesized
in short Okazaki fragments,
later joined by DNA ligase