- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Protein structure at action: bind transform release презентация
Содержание
- 1. Protein structure at action: bind transform release
- 2. BIND: repressors α- turn - α
- 3. DNA & RNA BINDING Zn- fingers Leu-zipper
- 4. -BINDING-INDUCED DEFORMATION MAKES REPRESSOR ACTIVE,
- 5. Immunoglobulin
- 6. Standard positions of active sites in protein folds
- 7. There are some with catalytic (Ser-protease) site
- 8. Preferential binding of TS: RIGID enzyme Catalysis:
- 9. Catalysis: stabilization of the transition state (TS)
- 10. Catalysis: stabilization of the transition state (TS)
- 11. Catalytic antibodies ABZYM = AntyBody enZYM
- 12. BIND ? TRANSFORM ? RELEASE: ENZYME Note: small active site chymotrypsin
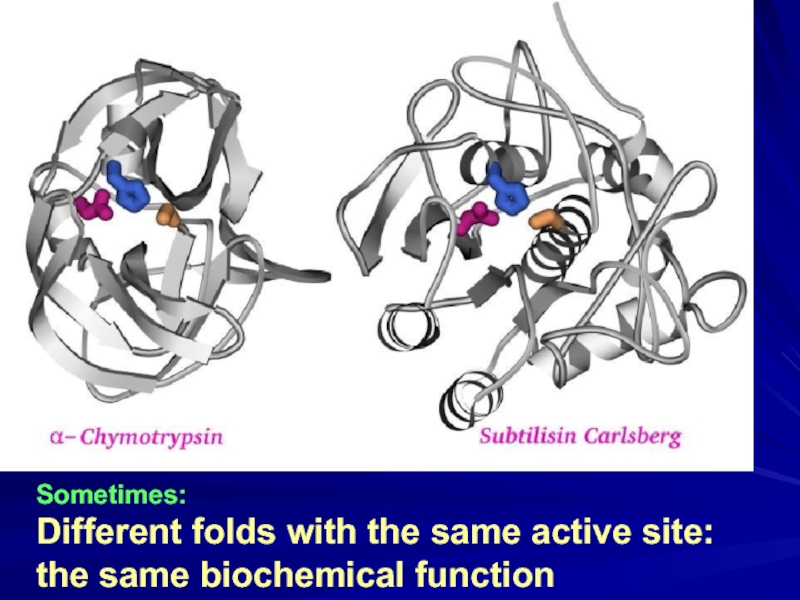
- 13. Sometimes: Different folds with the same active site: the same biochemical function
- 14. POST-TRANSLATIONAL MODIFICATION Sometimes, only the CHAIN
- 15. Chymotrypsin catalyses hydrolysis of a peptide Spontaneous hydrolysis: very slow
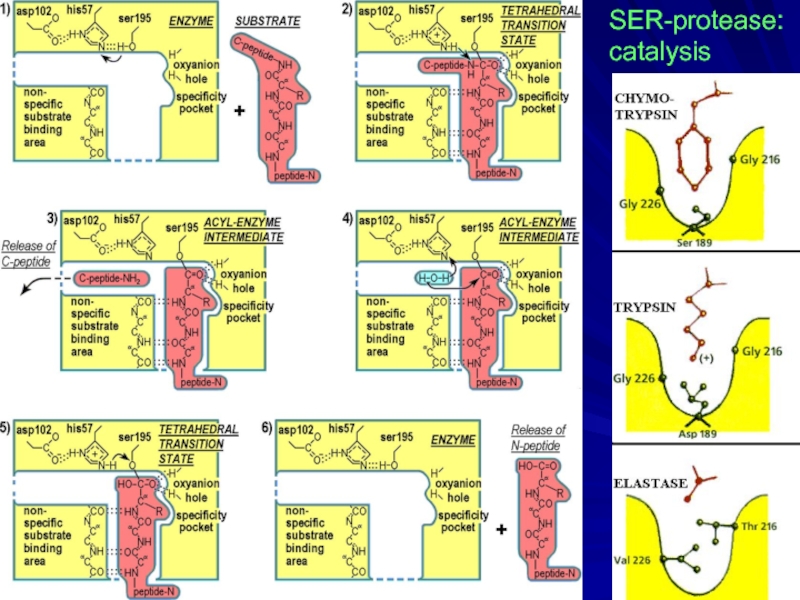
- 16. SER-protease: catalysis
- 17. CHYMOTRYPSIN ACTIVE SITE with INHIBITOR
- 18. Preferential binding of TS: RIGID enzyme
- 19. PROTEIN STRUCTURE AT ACTION: BIND ?
- 20. Induced fit model for enzyme catalysis. Daniel
- 21. MOTIONS
- 22. Double sieve: movement of substrate
- 23. Movement in two-domain enzyme: One conformation
- 24. Two-domain dehydrogenases: Universal NAD-binding domain; Individual substrate-binding domain
- 25. Movement in quaternary structure: Hemoglobin vs.
- 26. Kinesin : Linear cyclic motor
- 27. Kinesin : Linear cyclic motor
- 28. Sir Andrew Fielding Huxley (1917 – 2012) Nobel Prize 1963 Myosin "cross-bridges"
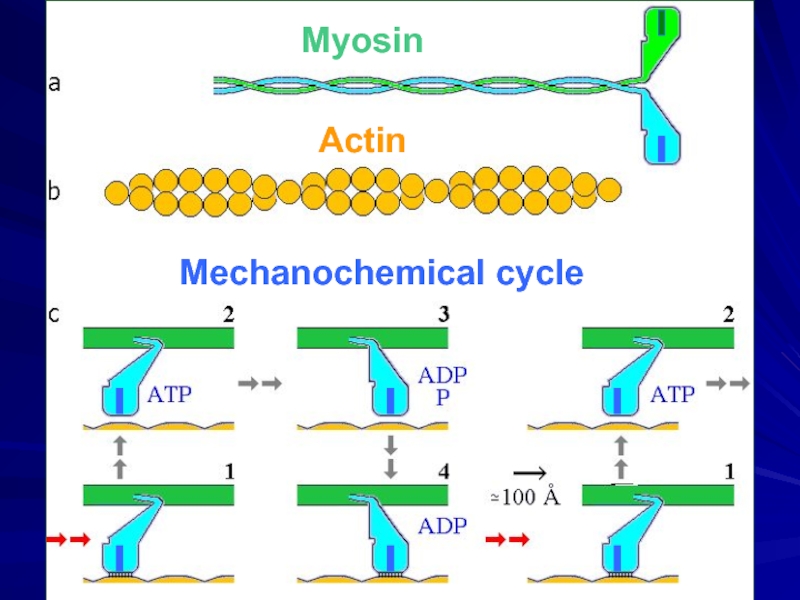
- 29. Механохимический цикл Миозин
- 30. Mechanochemical cycle Myosin Actin
- 31. structure from the X-ray data: Junge,
- 32. Engelbrecht & Junge, FEBS Lett. 414, 485
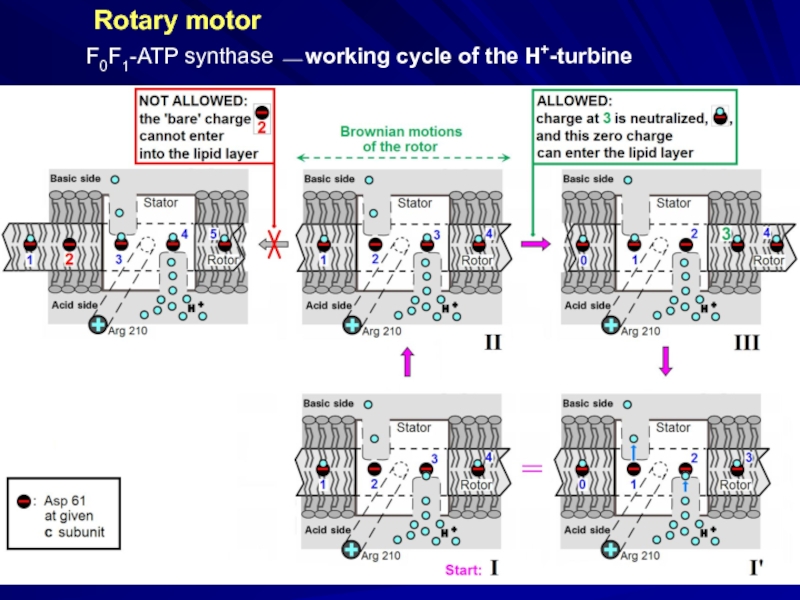
- 33. Rotary motor F0F1-ATP synthase ⎯ working cycle of the H+-turbine
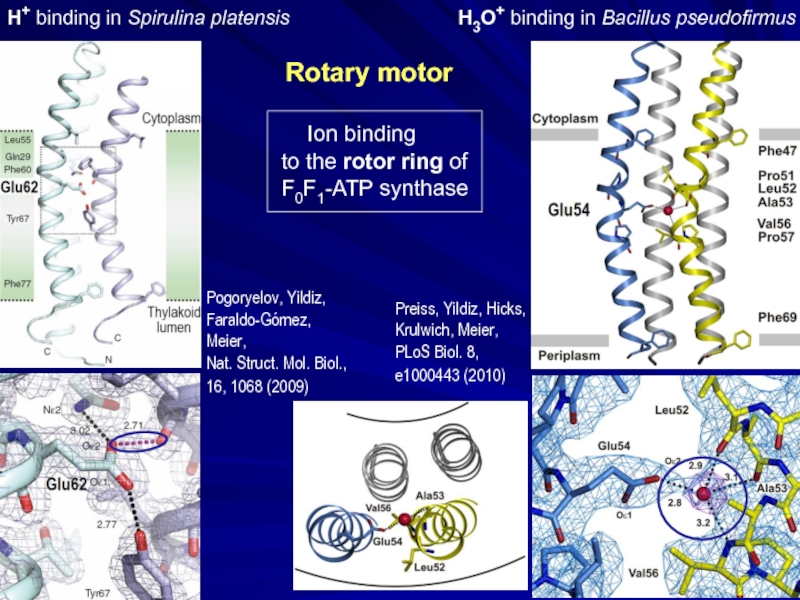
- 34. H3O+ binding in Bacillus pseudofirmus
- 35. SUMMARY of the course
- 36. PROTEIN PHYSICS Interactions
- 37. Intermediates & nuclei
- 38. Благодарю за внимание … товарищи офизевшие биологи!
- 39. Благодарю за внимание … товарищи офизевшие биологи!
Слайд 4-BINDING-INDUCED DEFORMATION
MAKES REPRESSOR ACTIVE, and IT BINDS TO DNA
BIND
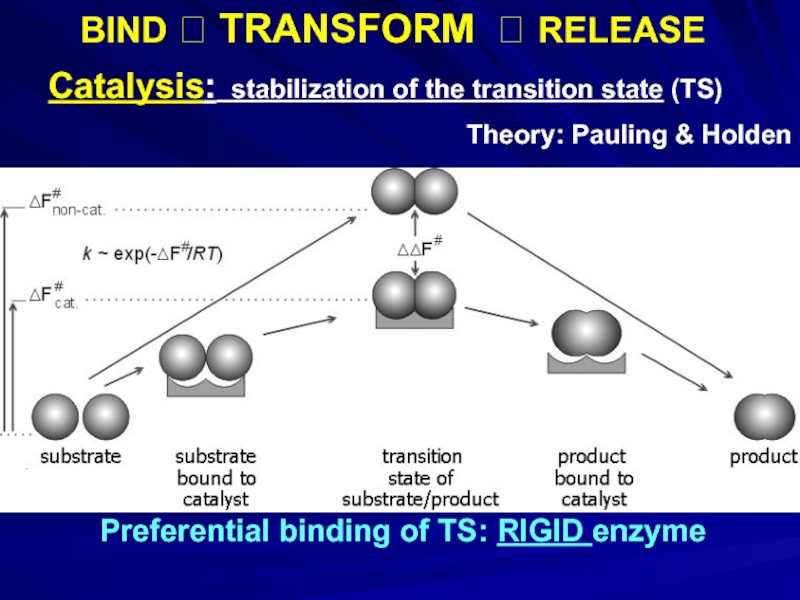
Слайд 8Preferential binding of TS: RIGID enzyme
Catalysis: stabilization of the transition state
Theory: Pauling & Holden
BIND ? TRANSFORM ? RELEASE
Слайд 9Catalysis: stabilization of the transition state (TS)
Theory: Pauling & Holden
Experimental verification:
______
__________
P
reputed
TS
Слайд 10Catalysis: stabilization of the transition state (TS)
Theory: Pauling & Holden
Experimental verification:
______
__________
P
reputed
TS
/
/
/
/
This
protein
engineering
reduces
the rate
by 1000000
Preferential
binding
of TS:
RIGID
enzyme
Слайд 11Catalytic antibodies
ABZYM = AntyBody enZYM
Antibodies
are
selected
to TS-like
molecule
Transition state (TS
Preferential
binding
of TS:
RIGID
enzyme
BIND ? TRANSFORM ? RELEASE
Suggested by Jencks in 1969
Done by Schultz and Lerner in 1994
Слайд 14POST-TRANSLATIONAL MODIFICATION
Sometimes, only the CHAIN CUT-INDUCED DEFORMATION
MAKES THE ENZYME ACTIVE
Chymotripsinogen
active
cat. site
non-active “cat. site”
CUT
Chymotripsin
⇒
Слайд 18Preferential binding of TS: RIGID enzyme
F = k1x1
Hooke’s & 2-nd Newton’s Energy is concentrated
laws in the softer body.
Effective catalysis: when
substrate is softer than protein
Kinetic energy cannot be stored for catalysis
Friction stops a molecule within
picoseconds:
m(dv/dt) = -(3πDη)v [Stokes law]
D – diameter; m ~ D3 – mass; η – viscosity
tkinet ≈ 10-13 sec × (D/nm)2 in water
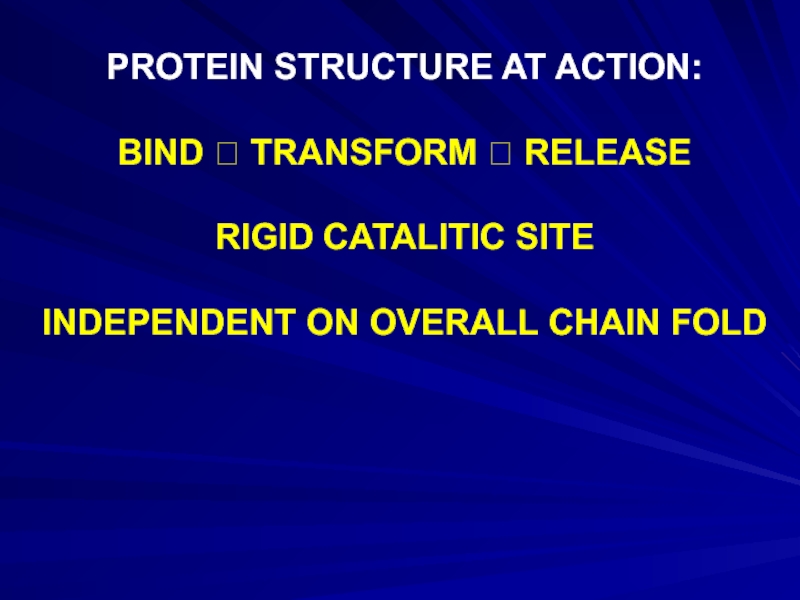
Слайд 19PROTEIN STRUCTURE AT ACTION:
BIND ? TRANSFORM ? RELEASE
RIGID CATALITIC SITE
INDEPENDENT
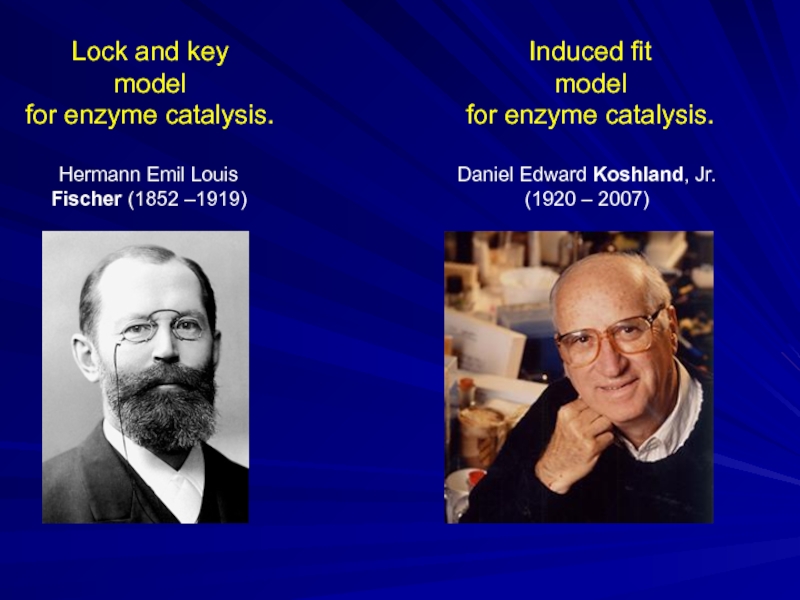
Слайд 20Induced fit
model
for enzyme catalysis.
Daniel Edward Koshland, Jr.
(1920 – 2007)
Hermann Emil Louis
Fischer (1852 –1919)
Lock and key
model
for enzyme catalysis.
Слайд 22Double sieve:
movement of substrate
from one active site to another
⇑
tRNAIle
Fersht A.R., Dingwall C.
Слайд 23Movement in two-domain enzyme:
One conformation for binding (and release),
another for
Induced fit
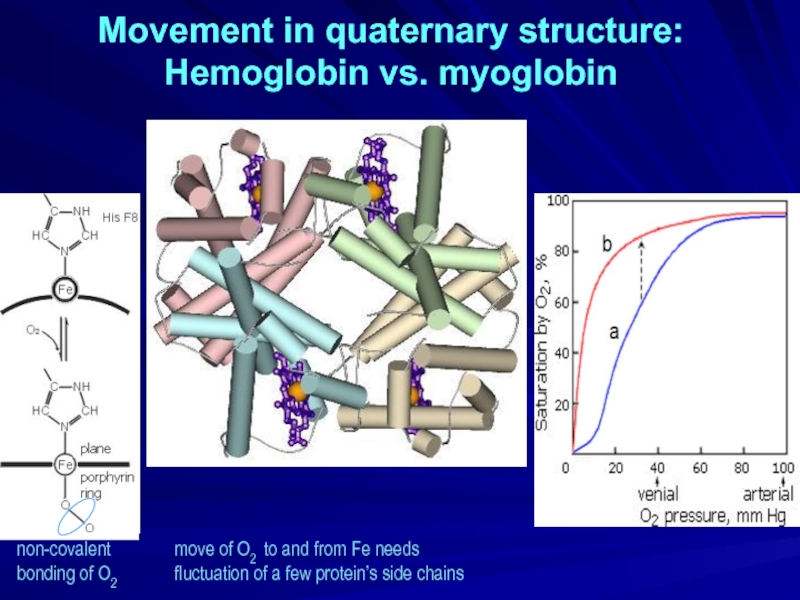
Слайд 25Movement in quaternary structure:
Hemoglobin vs. myoglobin
non-covalent
bonding of O2
move of
fluctuation of a few protein’s side chains
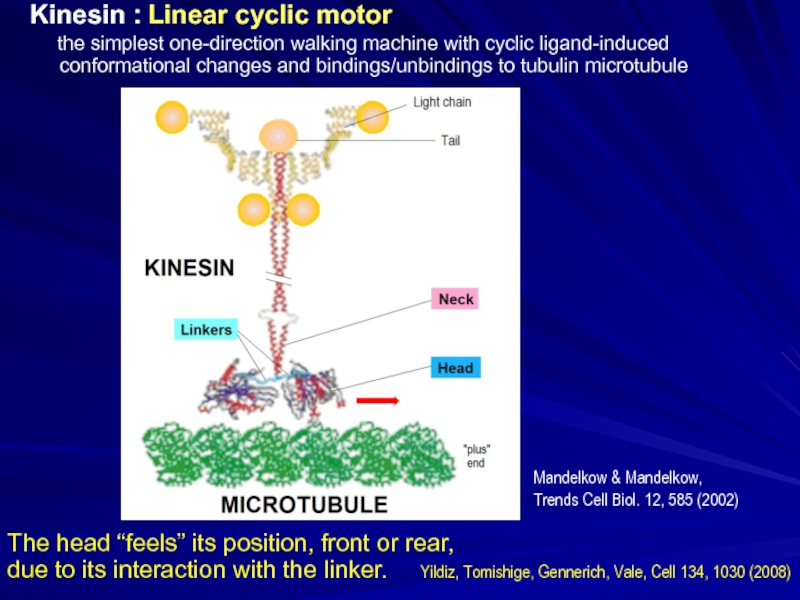
Слайд 26Kinesin : Linear cyclic motor
the simplest one-direction
Mandelkow & Mandelkow,
Trends Cell Biol. 12, 585 (2002)
The head “feels” its position, front or rear,
due to its interaction with the linker. Yildiz, Tomishige, Gennerich, Vale, Cell 134, 1030 (2008)
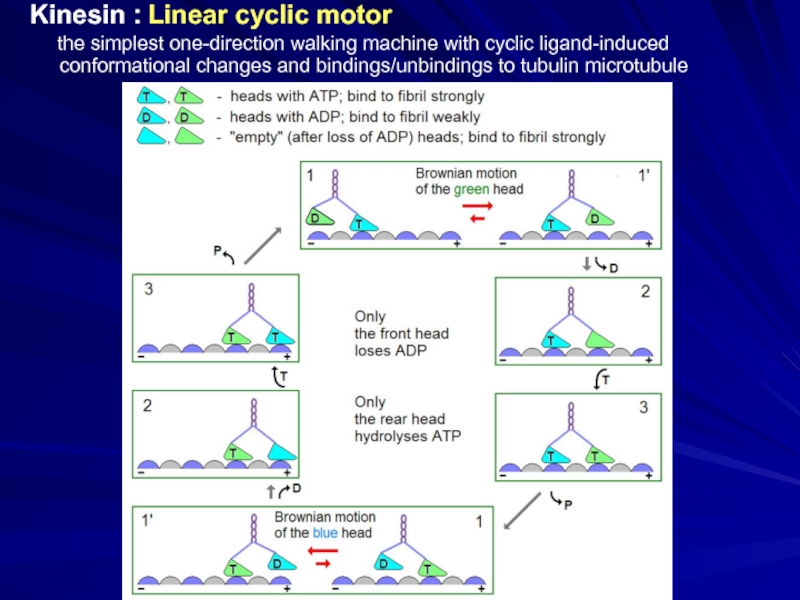
Слайд 27Kinesin : Linear cyclic motor
the simplest one-direction
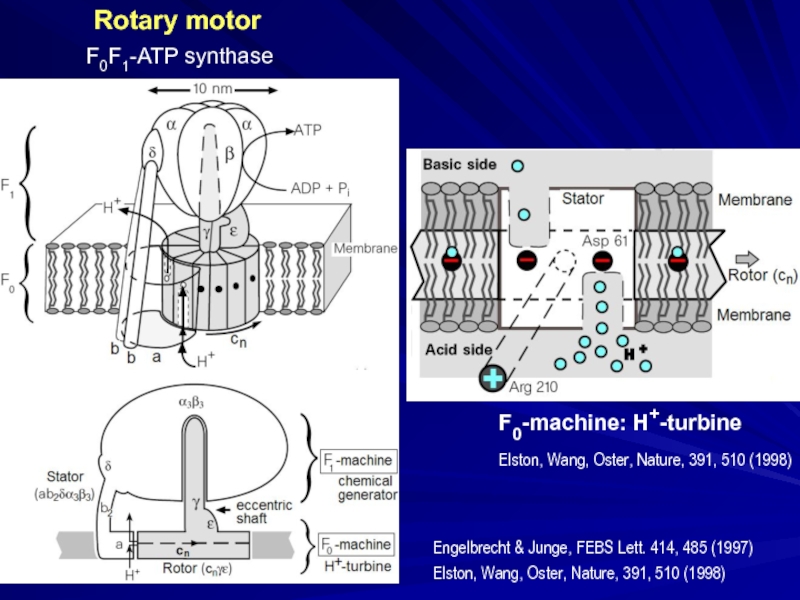
Слайд 31
structure from the X-ray data: Junge, Sielaff, Engelbrecht, Nature, 459, 364
Rotary motor
F0F1-ATP synthase
Слайд 32Engelbrecht & Junge, FEBS Lett. 414, 485 (1997)
Elston, Wang, Oster, Nature,
F0-machine: H+-turbine
Elston, Wang, Oster, Nature, 391, 510 (1998)
Acid side
Basic side
Rotary motor
F0F1-ATP synthase
Слайд 34
H3O+ binding in Bacillus pseudofirmus
Ion binding
to
F0F1-ATP synthase
H+ binding in Spirulina platensis
Rotary motor
Pogoryelov, Yildiz,
Faraldo-Gómez, Meier,
Nat. Struct. Mol. Biol.,
16, 1068 (2009)
Preiss, Yildiz, Hicks, Krulwich, Meier, PLoS Biol. 8, e1000443 (2010)