- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Immunophysiology of obesity презентация
Содержание
- 1. Immunophysiology of obesity
- 9. Adipose tissue depots occur throughout the body.
- 10. Adipose tissue constitutes an extremely active endocrine
- 11. Proteins secreted by the adipocyte. The list
- 12. WAT: white adipose tissue, SAA3: serum amyloid
- 14. Recent evidence has also pointed to the
- 15. Whereas macrophages in lean mice and
- 18. Role of the immune system in lean
- 20. • Macrophages play a significant role in
- 21. Feedback loop between the brain and adipose
- 22. While it has long been clear that
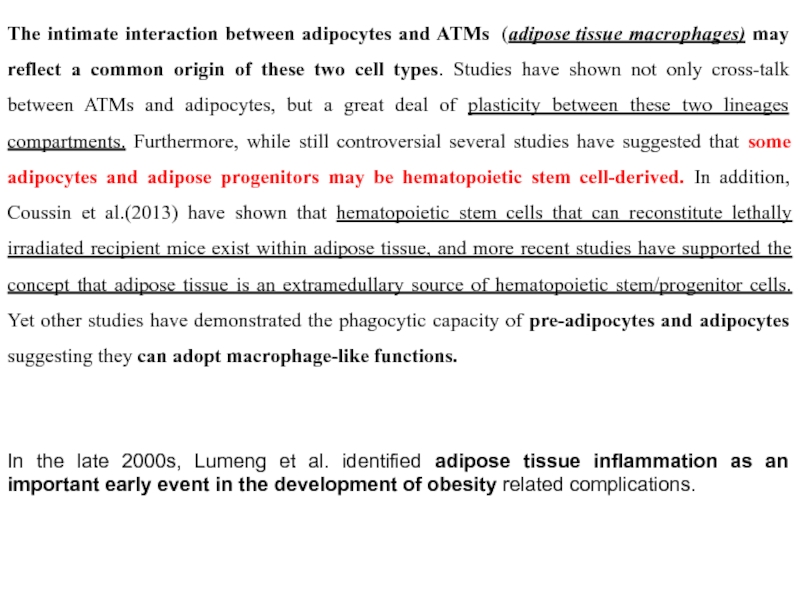
- 23. The intimate interaction between adipocytes and ATMs
- 24. In healthy adipose tissue, tissue remodeling is
- 28. a | Adipocytes and nerve cells. Parenchymal sympathetic nerve
- 29. Obesity increases the risk of developing a
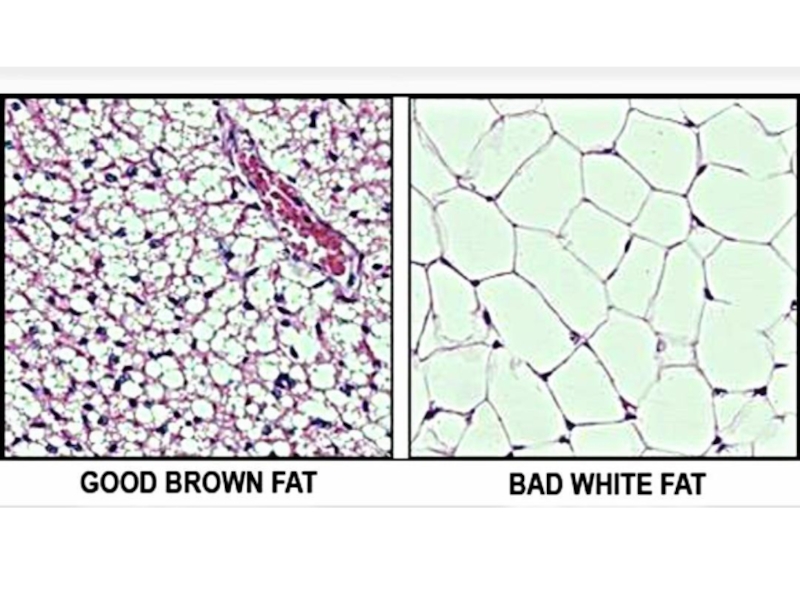
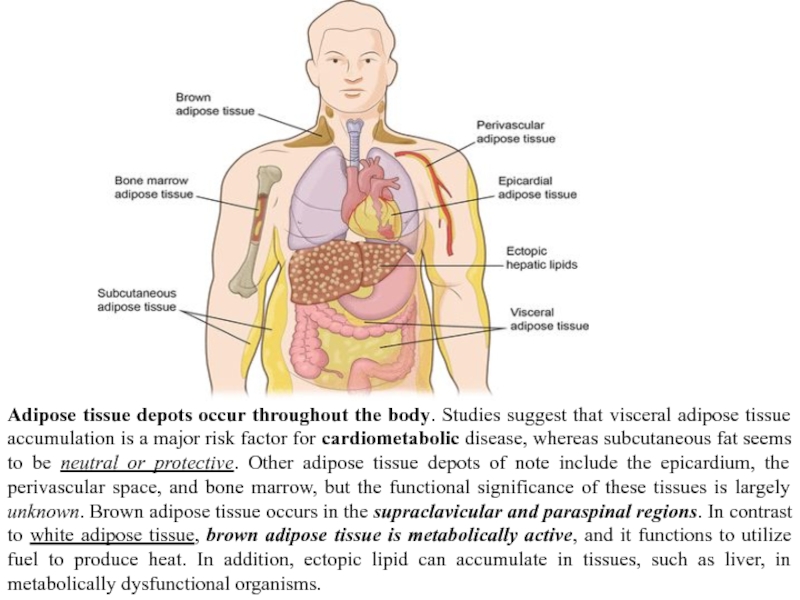
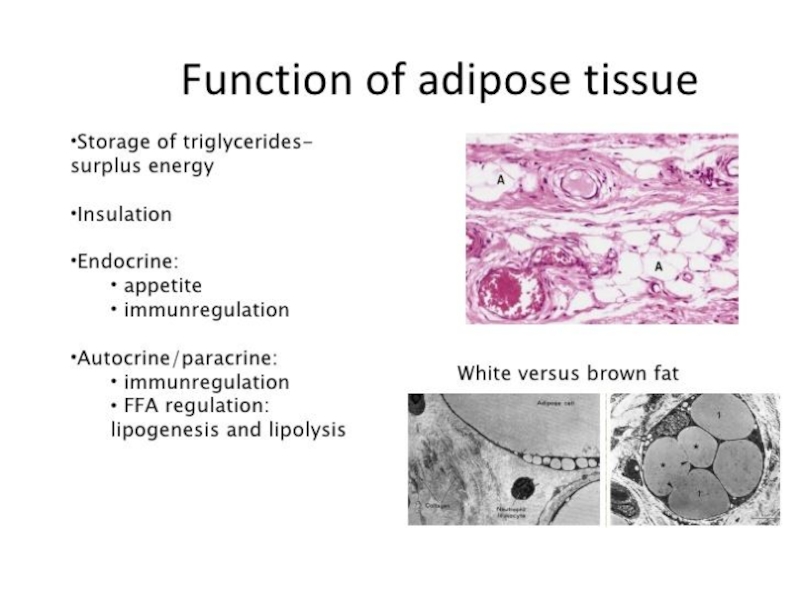
Слайд 9Adipose tissue depots occur throughout the body. Studies suggest that visceral
Слайд 10 Adipose tissue constitutes an extremely active endocrine organ with a network
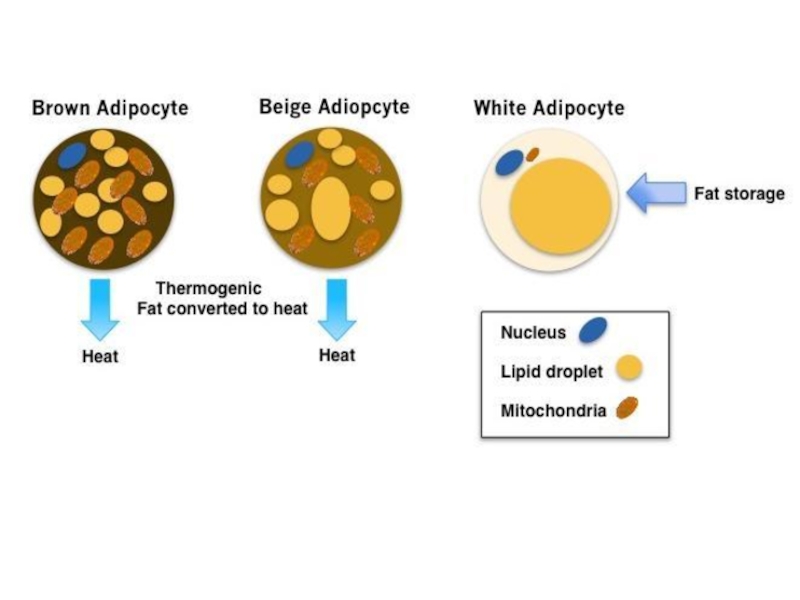
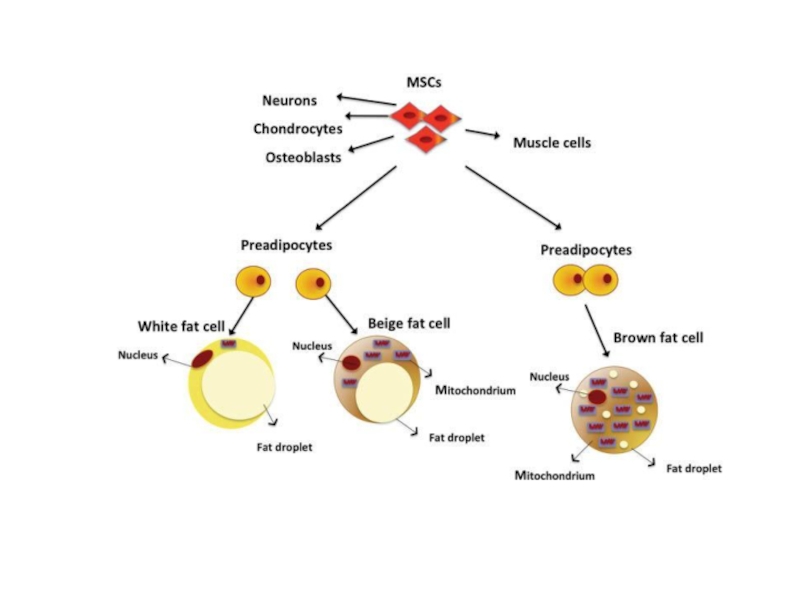
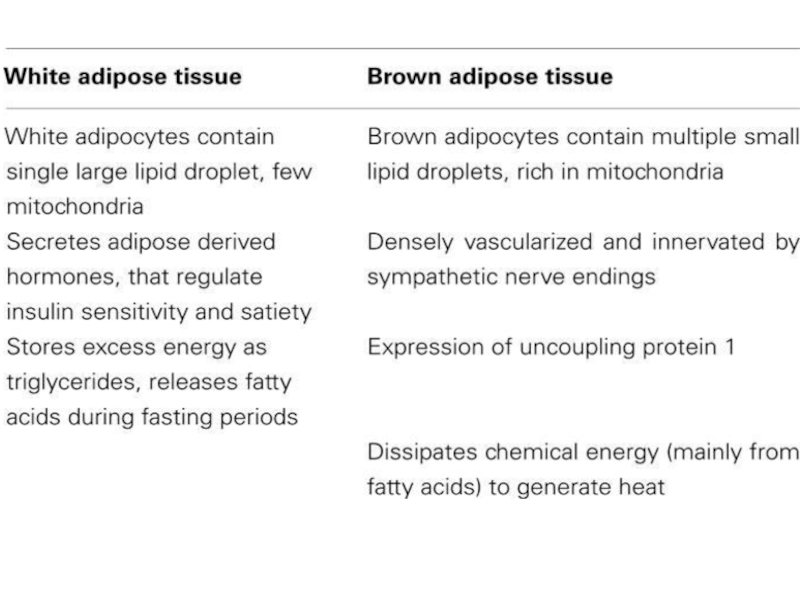
The mechanisms underlying the development of obesity and its associated comorbidities include the hypertrophy and/or hyperplasia of adipocytes, adipose tissue inflammation, impaired extracellular matrix remodeling, and fibrosis together with an altered secretion of adipokines. Recently, the potential role of brown and beige adipose tissue in the protection against obesity has been also recognized. In contrast to white adipocytes, which store energy in the form of fat, brown and beige fat cells display energy-dissipating capacity through the promotion of triacylglycerol clearance, glucose disposal, and generation of heat for thermogenesis. Identification of the morphological and molecular changes in white, beige, and brown adipose tissue during weight gain is of utmost relevance for the identification of pharmacological targets for the treatment of obesity and its associated metabolic diseases.
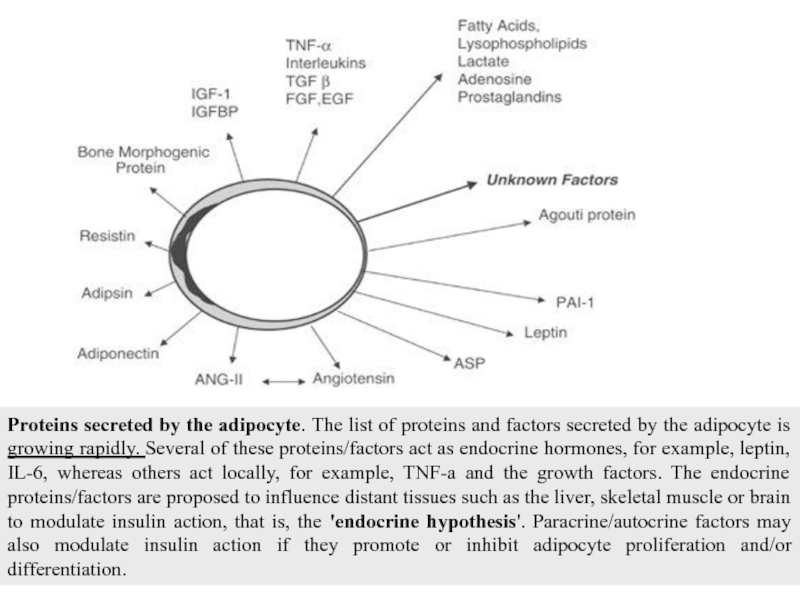
Слайд 11Proteins secreted by the adipocyte. The list of proteins and factors
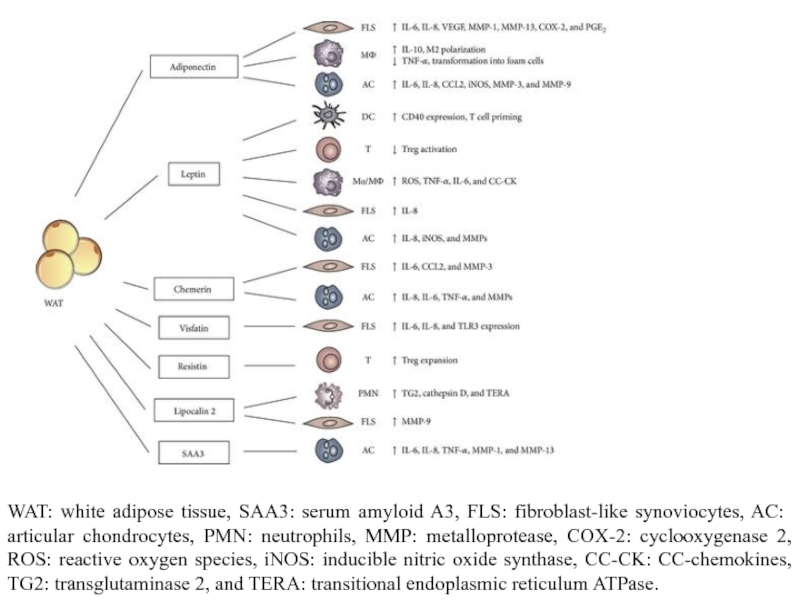
Слайд 12WAT: white adipose tissue, SAA3: serum amyloid A3, FLS: fibroblast-like synoviocytes,
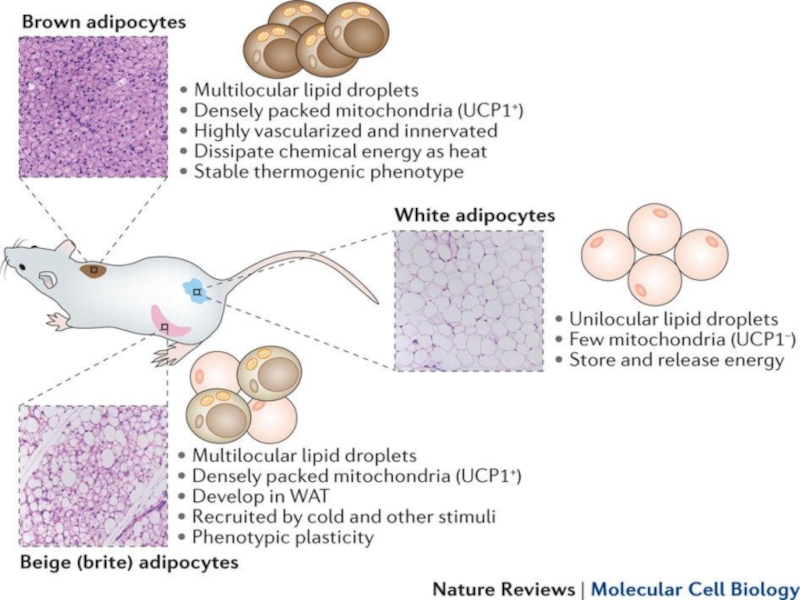
Слайд 14Recent evidence has also pointed to the heterogeneity of adipose tissue
Слайд 15
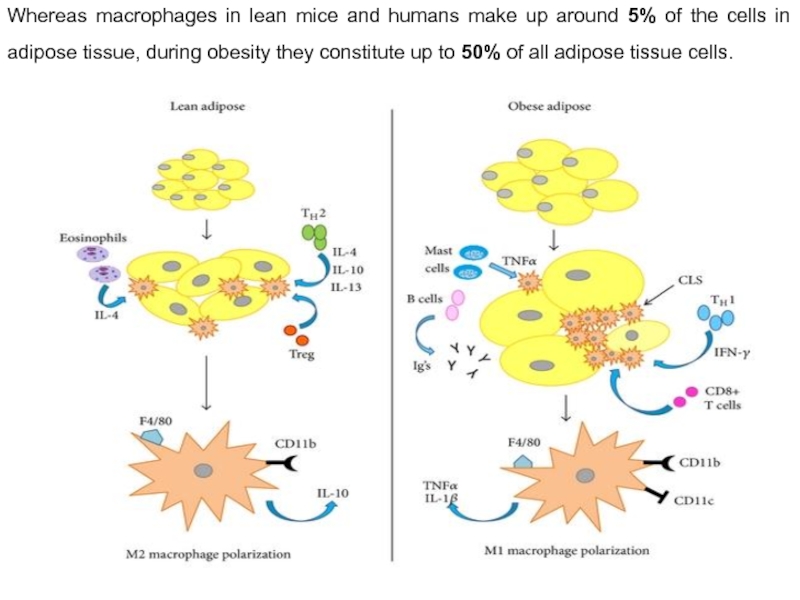
Whereas macrophages in lean mice and humans make up around 5%
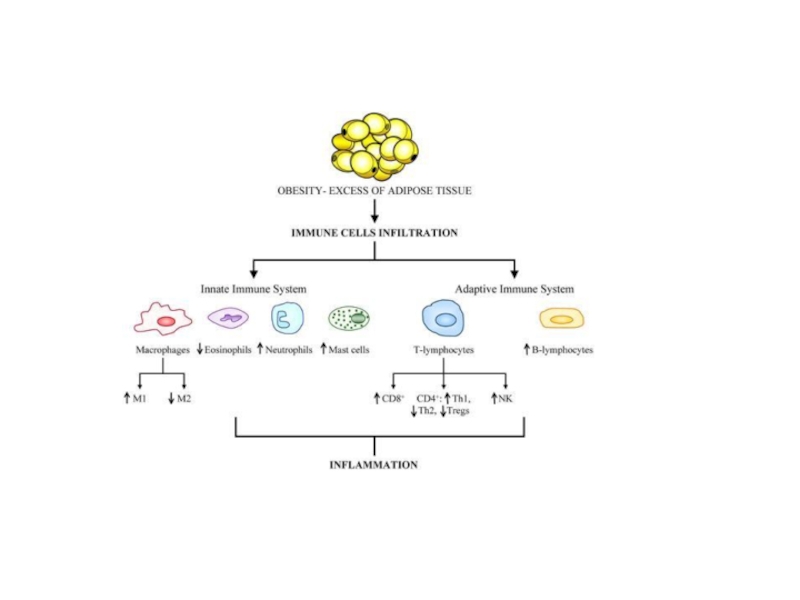
Слайд 18Role of the immune system in lean versus obese adipose tissue.
In lean adipose tissue, T-helper type 2 (TH2) cells produce anti-inflammatory cytokines such as interleukin (IL)-4, 10, and 13 which promote alternatively activated M2 macrophage polarization. M2 polarization is also induced by regulatory T cells (Tregs) and eosinophils via IL-4. M2 macrophages secrete other anti-inflammatory signals such as IL-10 which maintain insulin sensitivity within lean adipose tissue. Conversely, TH1 type cytokines such as interferon (IFN)-γ stimulate M1 macrophage polarization in obese adipose tissue. Other immune cells are also increased in obese adipose tissue which contribute to insulin resistance including mast cells, B cells, and immunoglobulins (Igs). CD8(+) T cells promote Adipose tissue macrophages (ATM) accumulation and proinflammatory gene expression and are also increased as well. Contrary to the lean state, in which ATMs are distributed throughout the adipose tissue exposing limited inflammatory properties, ATMs in obese adipose tissue are located around dead adipocytes and form so-called crown-like structures (CLSs) while displaying profound proinflammatory features. Macrophage presence in CLSs within obese adipose tissue has been directly linked with insulin resistance.
M1 macrophages are proinflammatory, secreting cytokines such as TNF-α and IL-1β. Macrophages are bone-marrow-derived myeloid cells hence both M1 and M2 macrophages express the myeloid cell surface markers F4/80 and CD11b. However, only the M1 population expresses the marker CD11c.
Слайд 20• Macrophages play a significant role in regulating adipose tissue functioning
• In addition to conventional functions such as clearing cellular debris and participating in tissue immune surveillance, lipid buffering is an important function of ATMs
• Obesity-induced inflammation, characterised by an elevated number of proinflammatory macrophages in adipose tissue, has been suggested to contribute to systemic insulin resistance
• Their origin, as well as a combination of peripheral changes and adipose tissue-derived stressors, probably contribute to ATM dysfunction and inflammatory traits during obesity
• Identification of transcriptional differences between ATMs from lean vs obese adipose tissue at several key points during the development of obesity and insulin resistance
may reveal upstream triggers, regulatory factors and intracellular pathways that shape ATM function
• Targeting metabolic capacity rather than the inflammatory phenotype of ATMs may hold potential to restore ATM function and adipose tissue homeostasis in obese individuals
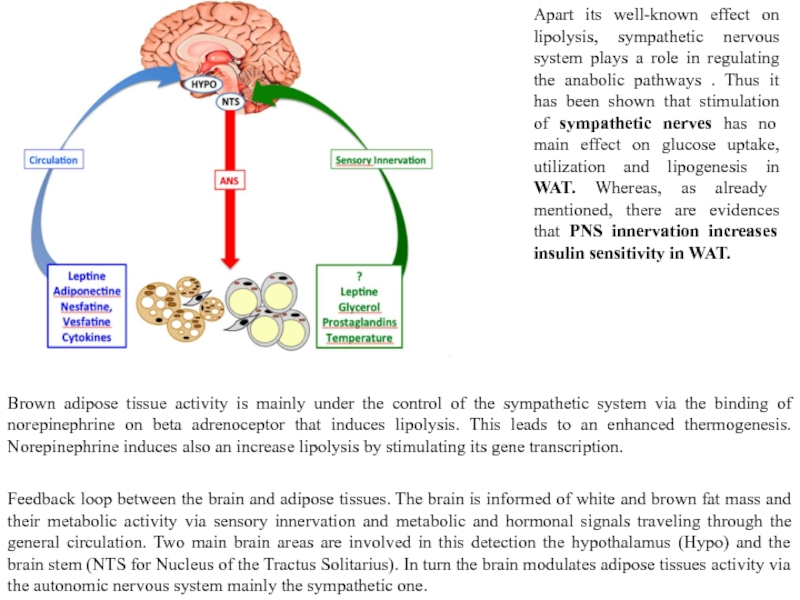
Слайд 21Feedback loop between the brain and adipose tissues. The brain is informed
Apart its well-known effect on lipolysis, sympathetic nervous system plays a role in regulating the anabolic pathways . Thus it has been shown that stimulation of sympathetic nerves has no main effect on glucose uptake, utilization and lipogenesis in WAT. Whereas, as already mentioned, there are evidences that PNS innervation increases insulin sensitivity in WAT.
Brown adipose tissue activity is mainly under the control of the sympathetic system via the binding of norepinephrine on beta adrenoceptor that induces lipolysis. This leads to an enhanced thermogenesis. Norepinephrine induces also an increase lipolysis by stimulating its gene transcription.
Слайд 22While it has long been clear that undernutrition can impair immunocompetence,
Work by both Spiegelman and Lumengetal demonstrate critical interactions between adipose tissue metabolism and the immune system, and the active role of adipose tissue macrophage (ATM) polarization in the progression of obesity. Lean individuals in a non-inflammatory state maintain a relatively low percentage (~10–15%) of resident ATMs. Healthy adipose tissue consists of uniformly distributed alternatively activated M2 macrophages. The M2 polarized state in healthy adipose tissue is maintained by eosinophils, which secrete IL-4. Polarized M2 ATMs secrete interleukin-10 (IL-10), which regulates glucose homeostasis within adipose tissue and systemic tissues including muscle. In turn adipose resident invariant natural killer Tcells (iNKT) induces the M2 phenotype through secretion of IL-10. Healthy adipocytes, in turn, secrete anti-inflammatory adipokines such as adiponectin, a hormone that acts synergistically with IL-4 to exert anti-inflammatory effects.
Слайд 23The intimate interaction between adipocytes and ATMs (adipose tissue macrophages) may reflect
In the late 2000s, Lumeng et al. identified adipose tissue inflammation as an important early event in the development of obesity related complications.
Слайд 24In healthy adipose tissue, tissue remodeling is accompanied by angiogenesis to
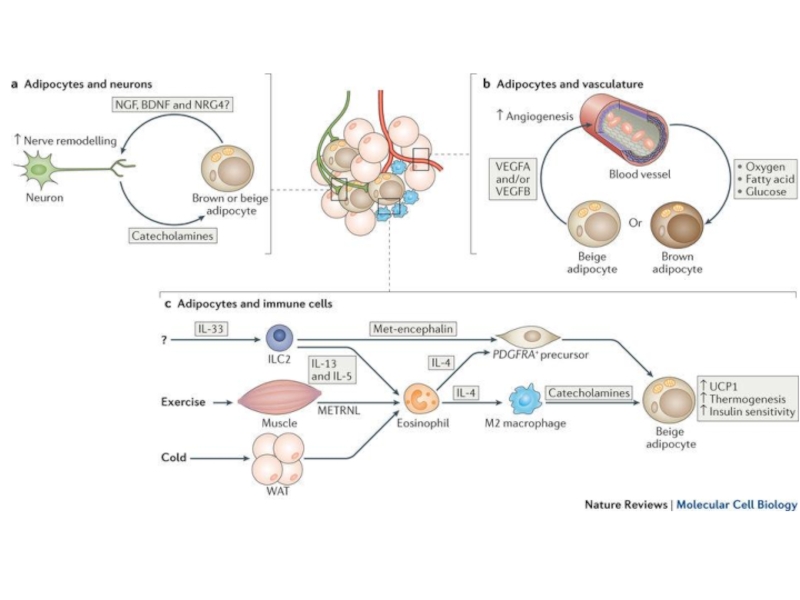
Слайд 28a | Adipocytes and nerve cells. Parenchymal sympathetic nerve fibres secrete catecholamines to
b | Brown and/or beige adipocytes and vasculature. Adipocytes secrete vascular endothelial growth factor (VEGF) to stimulate angiogenesis. The enhanced vasculature provides increased nutrition and oxygen to sustain thermogenesis in brown and beige adipocytes, thereby promoting energy expenditure and insulin sensitivity.
c | Immune cell regulation of beige adipocytes. Interleukin-33 (IL-33) (of unknown cellular source) activates group 2 innate lymphoid cells (ILC2s), which secrete IL-13 and IL-5. IL-5 activates eosinophils, which produce IL-4. IL-4, in turn, induces the differentiation of M2 macrophages, which provide a crucial source of catecholamines for beige fat activation. IL-4 (from eosinophils or ILC2s) also acts directly on platelet-derived growth factor receptor-α (PDGFRA)+ precursors to increase their proliferation and differentiation into beige adipocytes. Furthermore, ILC2s secrete Met-encephalin peptides to promote beige adipocyte differentiation. Meteorin-like protein (METRNL) secreted by muscle and WAT activates eosinophils and type II cytokine signalling to drive beige adipocyte development.
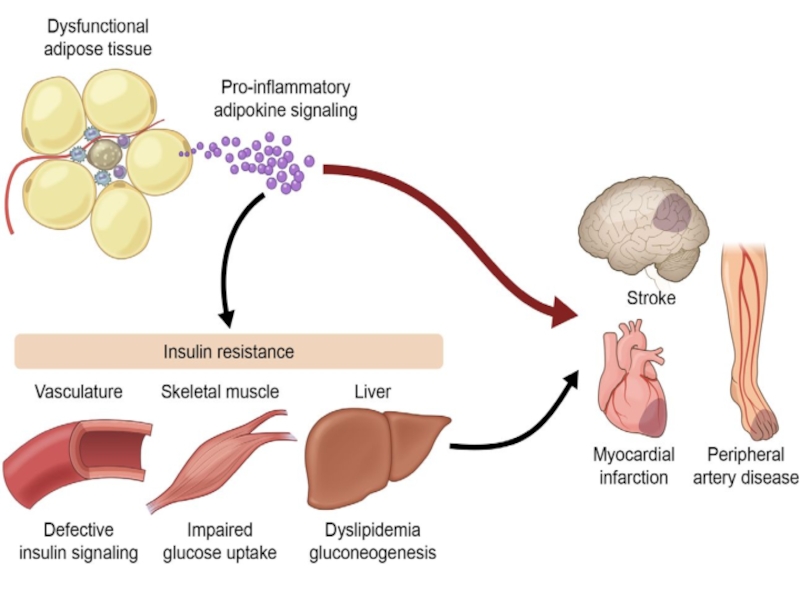
Слайд 29Obesity increases the risk of developing a number of serious health
Coronary heart disease
High blood pressure
Stroke
Type 2 diabetes
Cancer
Sleep apnea (внезапная остановка дыхания во сне)
Gallstones (желчный камень)
Osteoarthritis