- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
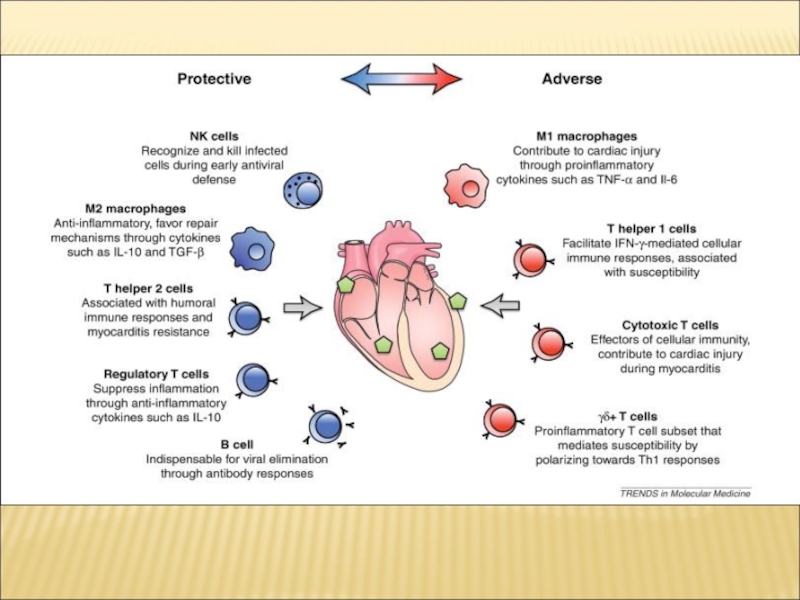
Immunophysiology of cardiovascular system презентация
Содержание
- 1. Immunophysiology of cardiovascular system
- 3. Origins and Dynamics of Resident Macrophage Subsets
- 5. Diverse role of macrophages in injury resolution.
- 7. Detailed hypertrophic and fibrotic signaling mechanisms between
- 8. Macrophages play an important role in regulating
- 9. Recent research findings suggest that macrophages contribute
- 11. 1.Double-stranded RNA (dsRNA). 2. CpG- oligodeoxynucleotides
- 14. The mammalian heart responds to stress through
- 15. Cardiotrophin-1 (CT1) is a cytokine in the
- 16. Proposed model of hCT1-mediated physiologic cardiac hypertrophy.
- 17. Interestingly, cardiomyocytes themselves can phagocytose latex particles
Слайд 3Origins and Dynamics of Resident Macrophage Subsets in the Adult Heart.
Resident
myocardial macrophages can be divided into two distinct pools. The most abundant populations originate from yolk sac progenitors, are able to self-renew, and are CCR2Ly6C. This population can be further dissected into MHCIIhi (shown in green) and MHCIIlo (shown in yellow). The second, smaller pool of resident cardiac macrophages is developmentally dependent on HSC-derived precursors and circulating monocytes for maintenance under steady state conditions. These cells can be further subdivided into CCR2 (shown in blue) and CCR2+ (shown in brown) populations. Although, LyC resident macrophages do not depend on circulating monocytes for maintenance under homeostatic conditions (left), during
cardiac stress (right), recruited Ly6C+ blood monocytes can differentiate into all resident cardiac macrophage subsets, which can subsequently expand and proliferate, thus restoring homeostasis within the macrophage compartment of the heart by two distinct mechanisms: local proliferation and HSC-derived engraftment.
cardiac stress (right), recruited Ly6C+ blood monocytes can differentiate into all resident cardiac macrophage subsets, which can subsequently expand and proliferate, thus restoring homeostasis within the macrophage compartment of the heart by two distinct mechanisms: local proliferation and HSC-derived engraftment.
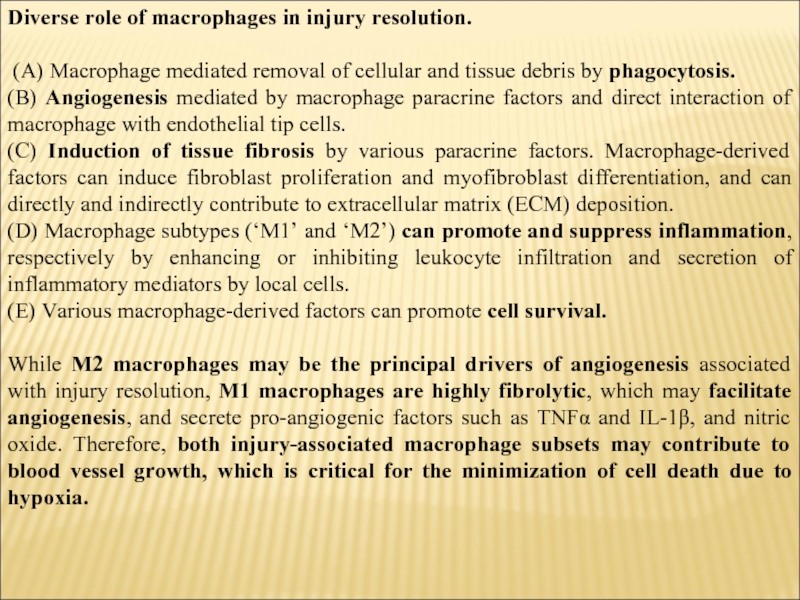
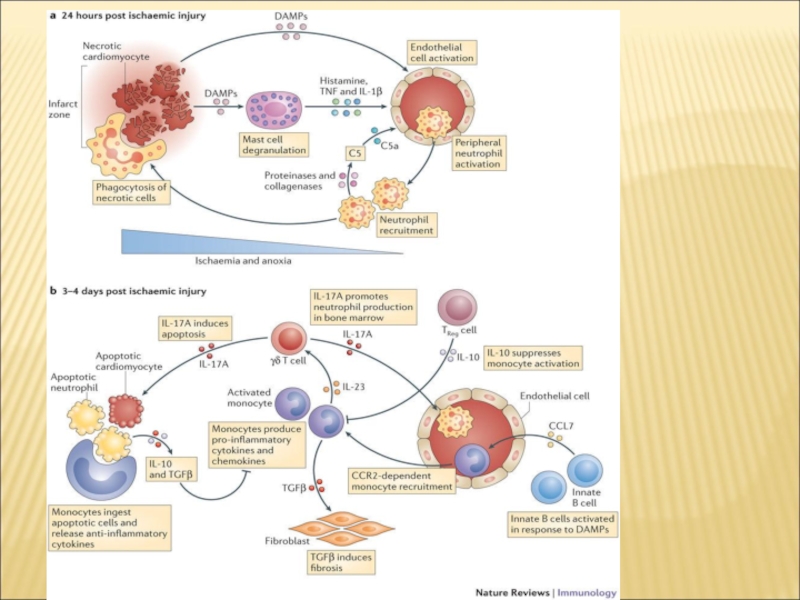
Слайд 5Diverse role of macrophages in injury resolution.
(A) Macrophage mediated removal
of cellular and tissue debris by phagocytosis.
(B) Angiogenesis mediated by macrophage paracrine factors and direct interaction of macrophage with endothelial tip cells.
(C) Induction of tissue fibrosis by various paracrine factors. Macrophage-derived factors can induce fibroblast proliferation and myofibroblast differentiation, and can directly and indirectly contribute to extracellular matrix (ECM) deposition.
(D) Macrophage subtypes (‘M1’ and ‘M2’) can promote and suppress inflammation, respectively by enhancing or inhibiting leukocyte infiltration and secretion of inflammatory mediators by local cells.
(E) Various macrophage-derived factors can promote cell survival.
While M2 macrophages may be the principal drivers of angiogenesis associated with injury resolution, M1 macrophages are highly fibrolytic, which may facilitate angiogenesis, and secrete pro-angiogenic factors such as TNFα and IL-1β, and nitric oxide. Therefore, both injury-associated macrophage subsets may contribute to blood vessel growth, which is critical for the minimization of cell death due to hypoxia.
(B) Angiogenesis mediated by macrophage paracrine factors and direct interaction of macrophage with endothelial tip cells.
(C) Induction of tissue fibrosis by various paracrine factors. Macrophage-derived factors can induce fibroblast proliferation and myofibroblast differentiation, and can directly and indirectly contribute to extracellular matrix (ECM) deposition.
(D) Macrophage subtypes (‘M1’ and ‘M2’) can promote and suppress inflammation, respectively by enhancing or inhibiting leukocyte infiltration and secretion of inflammatory mediators by local cells.
(E) Various macrophage-derived factors can promote cell survival.
While M2 macrophages may be the principal drivers of angiogenesis associated with injury resolution, M1 macrophages are highly fibrolytic, which may facilitate angiogenesis, and secrete pro-angiogenic factors such as TNFα and IL-1β, and nitric oxide. Therefore, both injury-associated macrophage subsets may contribute to blood vessel growth, which is critical for the minimization of cell death due to hypoxia.
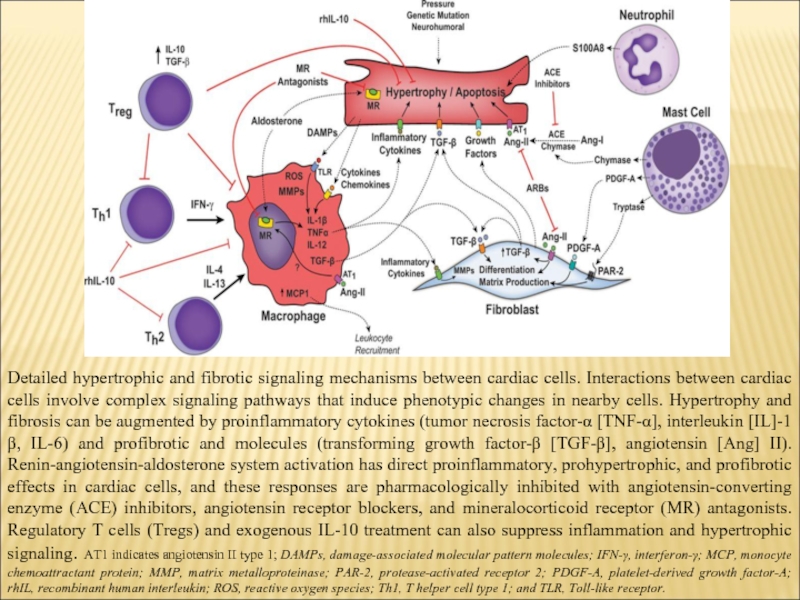
Слайд 7Detailed hypertrophic and fibrotic signaling mechanisms between cardiac cells. Interactions between
cardiac cells involve complex signaling pathways that induce phenotypic changes in nearby cells. Hypertrophy and fibrosis can be augmented by proinflammatory cytokines (tumor necrosis factor-α [TNF-α], interleukin [IL]-1β, IL-6) and profibrotic and molecules (transforming growth factor-β [TGF-β], angiotensin [Ang] II). Renin-angiotensin-aldosterone system activation has direct proinflammatory, prohypertrophic, and profibrotic effects in cardiac cells, and these responses are pharmacologically inhibited with angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor (MR) antagonists. Regulatory T cells (Tregs) and exogenous IL-10 treatment can also suppress inflammation and hypertrophic signaling. AT1 indicates angiotensin II type 1; DAMPs, damage-associated molecular pattern molecules; IFN-γ, interferon-γ; MCP, monocyte chemoattractant protein; MMP, matrix metalloproteinase; PAR-2, protease-activated receptor 2; PDGF-A, platelet-derived growth factor-A; rhIL, recombinant human interleukin; ROS, reactive oxygen species; Th1, T helper cell type 1; and TLR, Toll-like receptor.
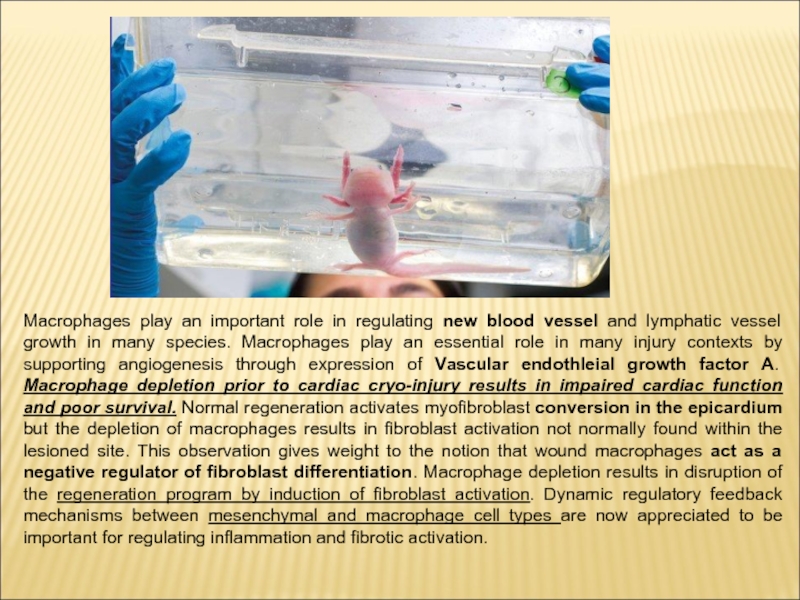
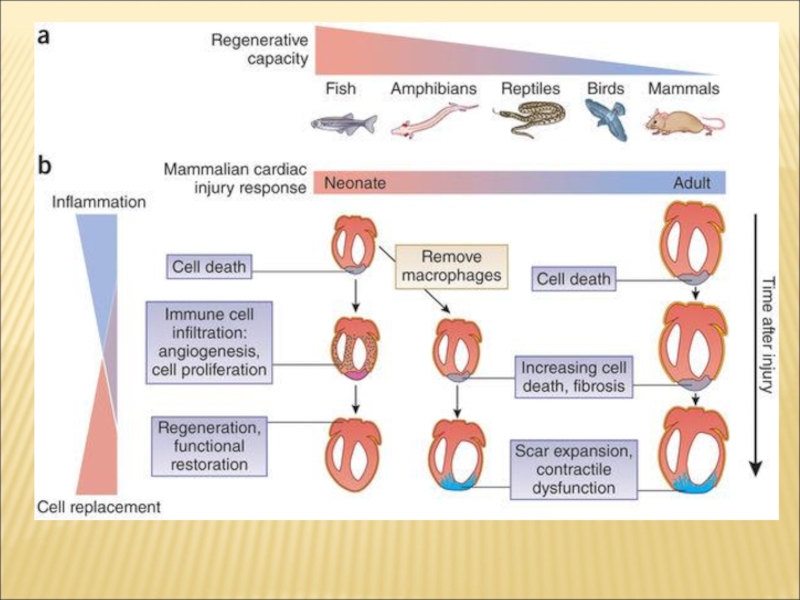
Слайд 8Macrophages play an important role in regulating new blood vessel and
lymphatic vessel growth in many species. Macrophages play an essential role in many injury contexts by supporting angiogenesis through expression of Vascular endothleial growth factor A. Macrophage depletion prior to cardiac cryo-injury results in impaired cardiac function and poor survival. Normal regeneration activates myofibroblast conversion in the epicardium but the depletion of macrophages results in fibroblast activation not normally found within the lesioned site. This observation gives weight to the notion that wound macrophages act as a negative regulator of fibroblast differentiation. Macrophage depletion results in disruption of the regeneration program by induction of fibroblast activation. Dynamic regulatory feedback mechanisms between mesenchymal and macrophage cell types are now appreciated to be important for regulating inflammation and fibrotic activation.
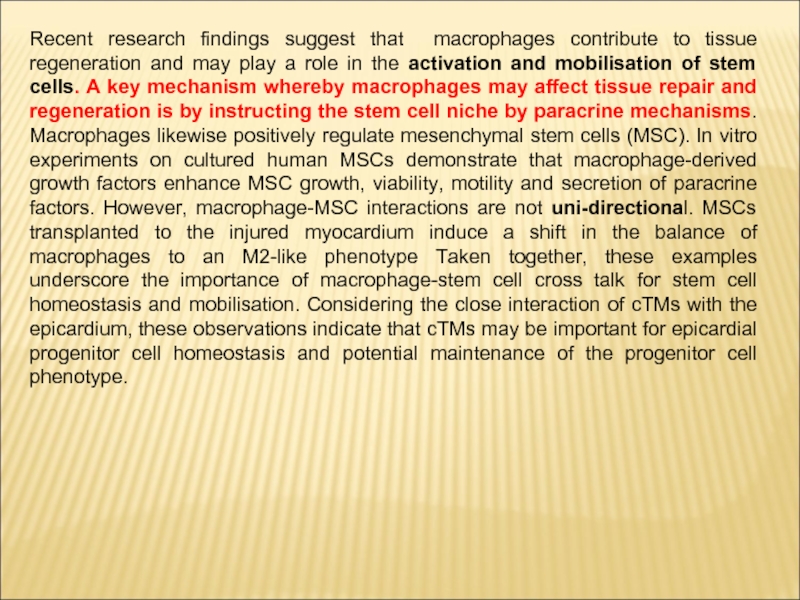
Слайд 9Recent research findings suggest that macrophages contribute to tissue regeneration and
may play a role in the activation and mobilisation of stem cells. A key mechanism whereby macrophages may affect tissue repair and regeneration is by instructing the stem cell niche by paracrine mechanisms. Macrophages likewise positively regulate mesenchymal stem cells (MSC). In vitro experiments on cultured human MSCs demonstrate that macrophage-derived growth factors enhance MSC growth, viability, motility and secretion of paracrine factors. However, macrophage-MSC interactions are not uni-directional. MSCs transplanted to the injured myocardium induce a shift in the balance of macrophages to an M2-like phenotype Taken together, these examples underscore the importance of macrophage-stem cell cross talk for stem cell homeostasis and mobilisation. Considering the close interaction of cTMs with the epicardium, these observations indicate that cTMs may be important for epicardial progenitor cell homeostasis and potential maintenance of the progenitor cell phenotype.
Слайд 11
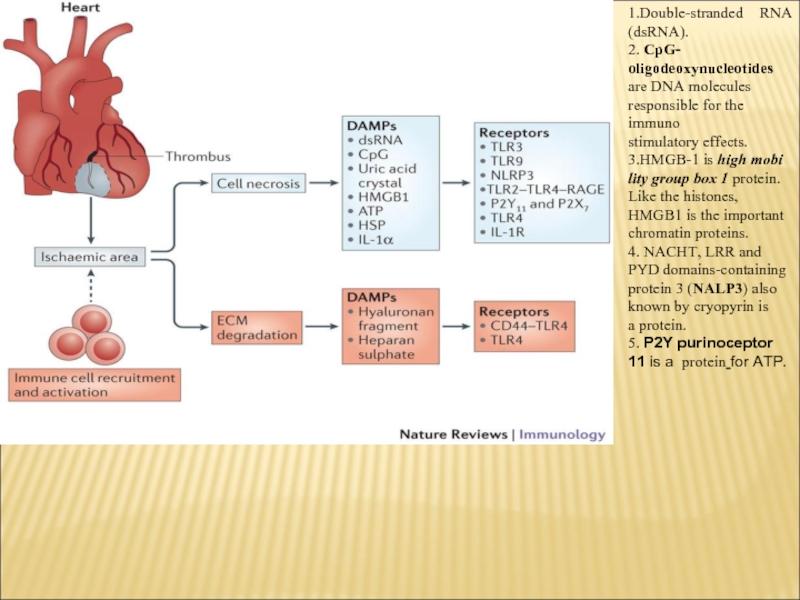
1.Double-stranded RNA (dsRNA).
2. CpG- oligodeoxynucleotides
are DNA molecules responsible for the immuno
stimulatory effects.
3.HMGB-1 is
high mobi lity group box 1 protein. Like the histones, HMGB1 is the important chromatin proteins.
4. NACHT, LRR and PYD domains-containing protein 3 (NALP3) also known by cryopyrin is a protein.
5. P2Y purinoceptor 11 is a protein for ATP.
4. NACHT, LRR and PYD domains-containing protein 3 (NALP3) also known by cryopyrin is a protein.
5. P2Y purinoceptor 11 is a protein for ATP.
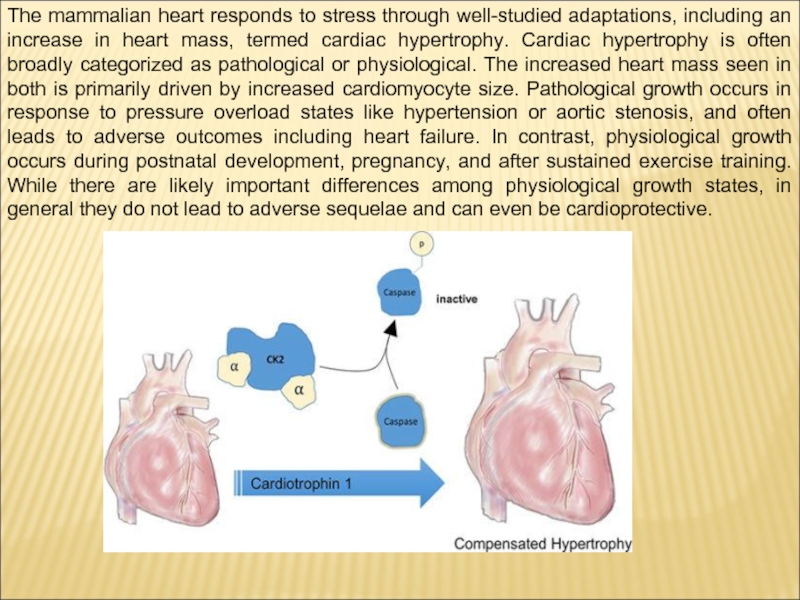
Слайд 14The mammalian heart responds to stress through well-studied adaptations, including an
increase in heart mass, termed cardiac hypertrophy. Cardiac hypertrophy is often broadly categorized as pathological or physiological. The increased heart mass seen in both is primarily driven by increased cardiomyocyte size. Pathological growth occurs in response to pressure overload states like hypertension or aortic stenosis, and often leads to adverse outcomes including heart failure. In contrast, physiological growth occurs during postnatal development, pregnancy, and after sustained exercise training. While there are likely important differences among physiological growth states, in general they do not lead to adverse sequelae and can even be cardioprotective.
Слайд 15Cardiotrophin-1 (CT1) is a cytokine in the interleukin-6 family and is
known to induce cardiac hypertrophy. Initially, CT1 was thought to drive pathological hypertrophy but was found to promote cell survival and induce proliferation of embryonic cardiomyocytes. In a recent paper in Cell Research, Abdul-Ghani et al. (2017) show that CT1 causes a reversible, protective form of cardiomyocyte hypertrophy.
Cardiotrophin 1 (CT-1) stimulates the production of interleukin 6 (IL-6). This suggests that at least in some pathological situations CT-1 might represent an immunomodulator regulating cytokine-induced gene products.
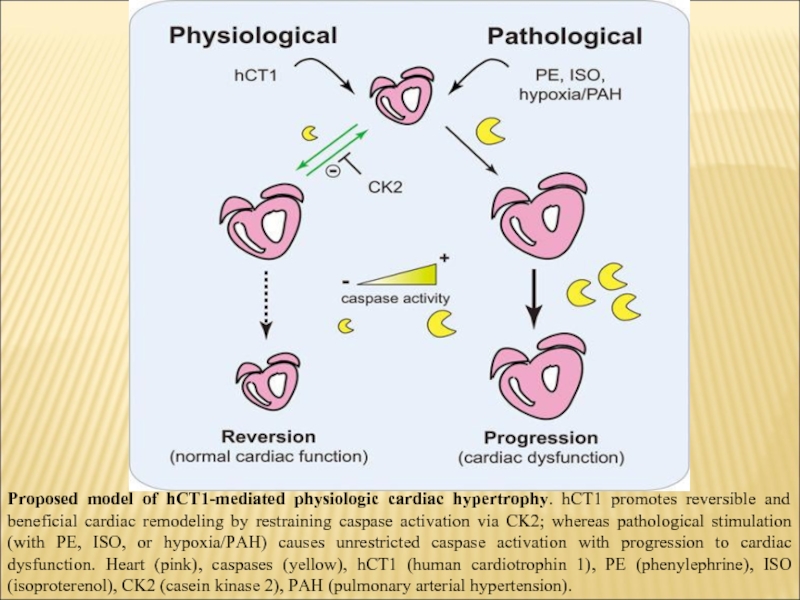
Слайд 16Proposed model of hCT1-mediated physiologic cardiac hypertrophy. hCT1 promotes reversible and
beneficial cardiac remodeling by restraining caspase activation via CK2; whereas pathological stimulation (with PE, ISO, or hypoxia/PAH) causes unrestricted caspase activation with progression to cardiac dysfunction. Heart (pink), caspases (yellow), hCT1 (human cardiotrophin 1), PE (phenylephrine), ISO (isoproterenol), CK2 (casein kinase 2), PAH (pulmonary arterial hypertension).
Слайд 17Interestingly, cardiomyocytes themselves can phagocytose latex particles in vitro and potentially
cardiomyocyte debris in vivo, the latter of which may have an important role in the developing heart. Recently, myofibroblasts were identified as another non-professional phagocyte that were capable of engulf-ing apoptotic cardiomyocytes. While the presence of professional cardiac phagocytes, such as macrophages, minimalizes the necessity for non-professional phagocytes, such as myofibroblasts, to phagocytose a dying neighbor, the contribution of non-professional phagocytes to the clearance of apoptotic and necrotic debris is underexplored and whether these cells cooperate with macrophages in the heart to promote cardiac repair requires additional study.