- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Formation of the two-layered blastoderm of the chick embryo презентация
Содержание
- 1. Formation of the two-layered blastoderm of the chick embryo
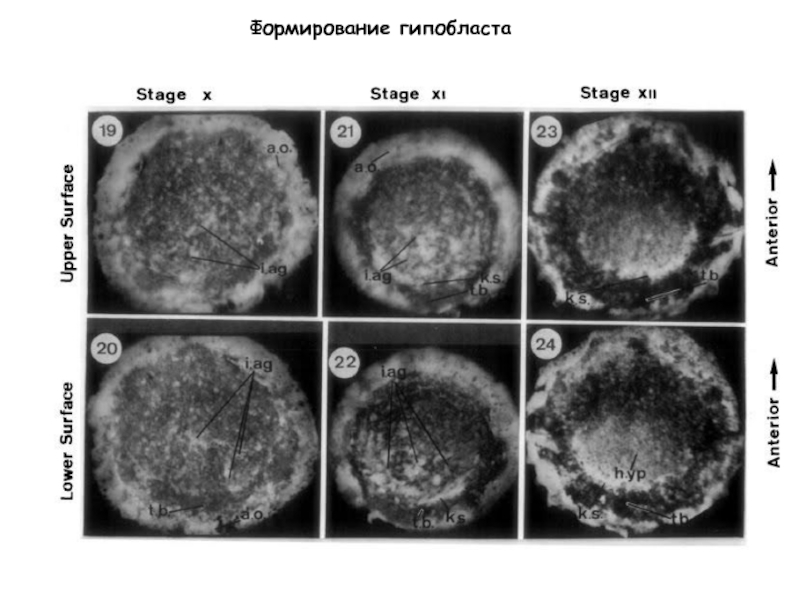
- 2. Stage XIII Stage XIV Stage 2 (HH)
- 3. Figure 11.9. Formation of the two-layered blastoderm
- 4. Fig. 1. a: SEM of the ventral
- 5. Figure 1. Hypoblast and Endoblast in Early
- 7. Движения «полонеза» до (А) и после (Б)
- 9. FIGURE 2. A series of frames from
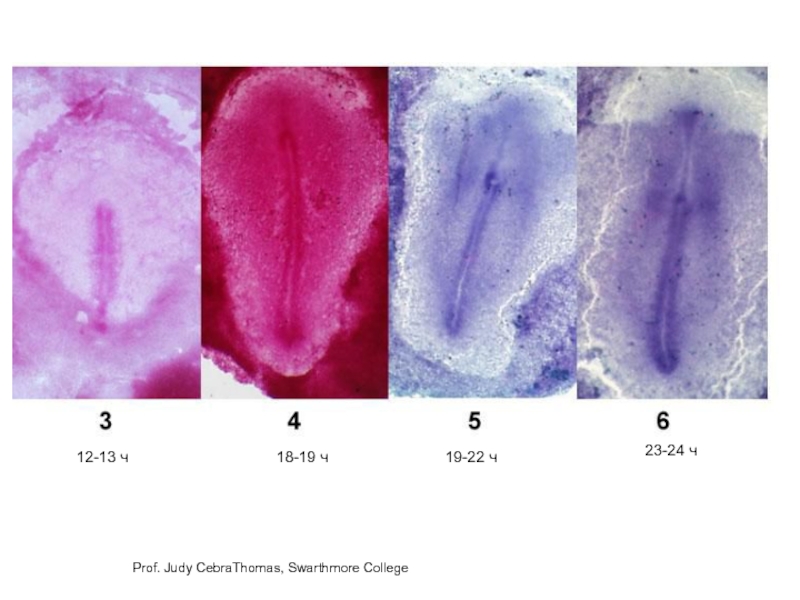
- 10. Prof. Judy CebraThomas, Swarthmore College 12-13 ч 18-19 ч 19-22 ч 23-24 ч
- 11. ПП появляется на ст. 2 в
- 12. FIGURE 3. (A) Scanning electron micrograph of
- 14. Figure 1. (A) General structures of
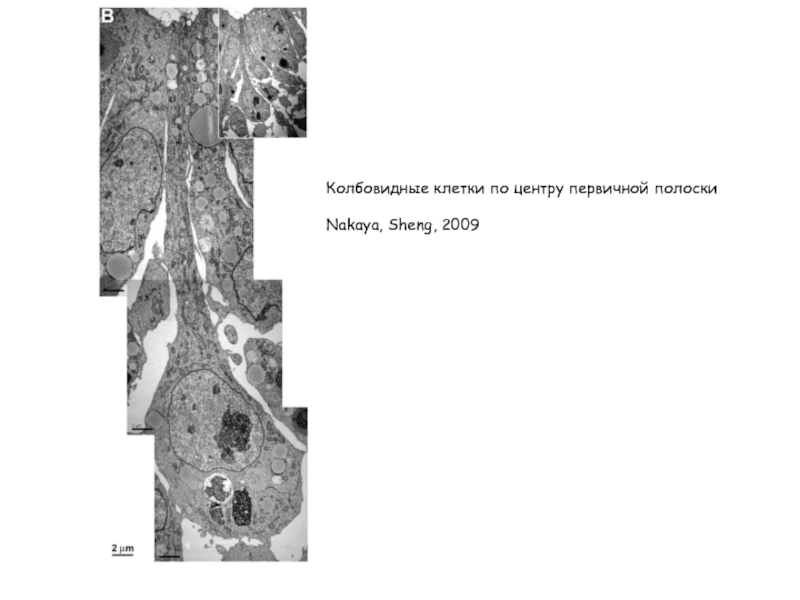
- 15. Колбовидные клетки по центру первичной полоски Nakaya, Sheng, 2009
- 16. Figure 1 Cellular aspects of EMT. (i)
- 17. Figure 3 Primary EMTs give rise to
- 18. Fig. 3. a: SEM of a Stage
- 19. Fig. 4. a: SEM of a stage
- 20. Fig. 5. a: SEM of a transverse
- 21. Fig. 12. Transverse sections of chick embryos
- 22. Механизмы удлинения ПП : конвергенция-растяжение
- 23. Fig. 10 Avian gastrulation begins with formation
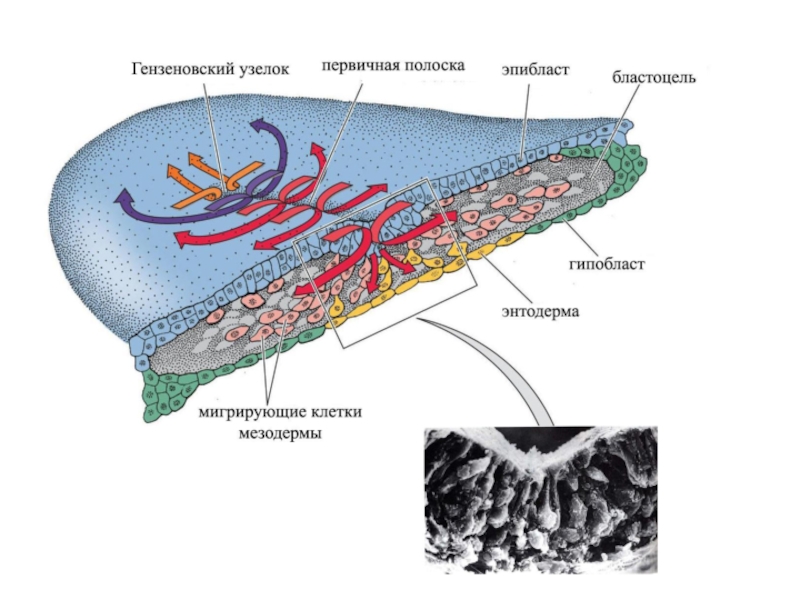
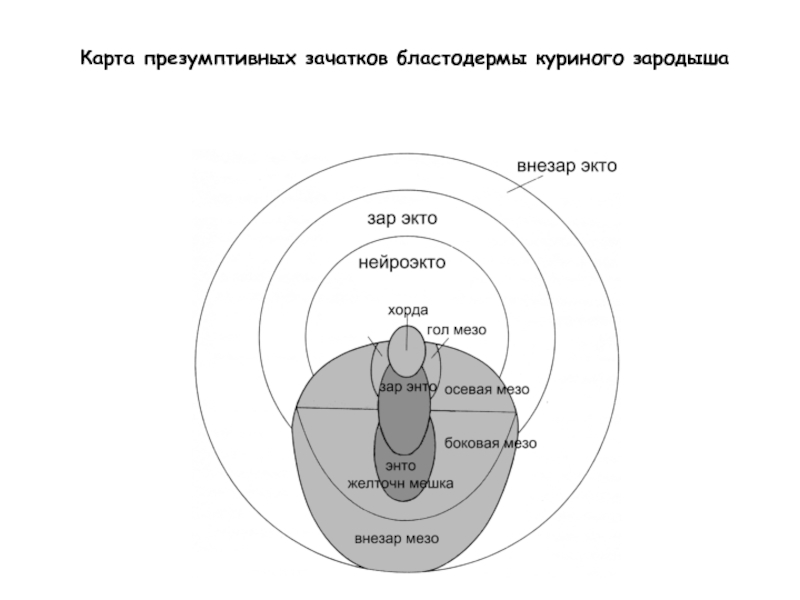
- 24. Карта презумптивных зачатков бластодермы куриного зародыша
- 25. Клетки проспективной мезодермы претерпевают ингрессию через ПП
- 26. Fig. 9. SEM of the ventral surface
- 27. Fig. 10. SEM of the ventral surface
- 28. FIGURE 5. A series of frames from
- 29. FIGURE 4. Fate mapping by following fluorescent
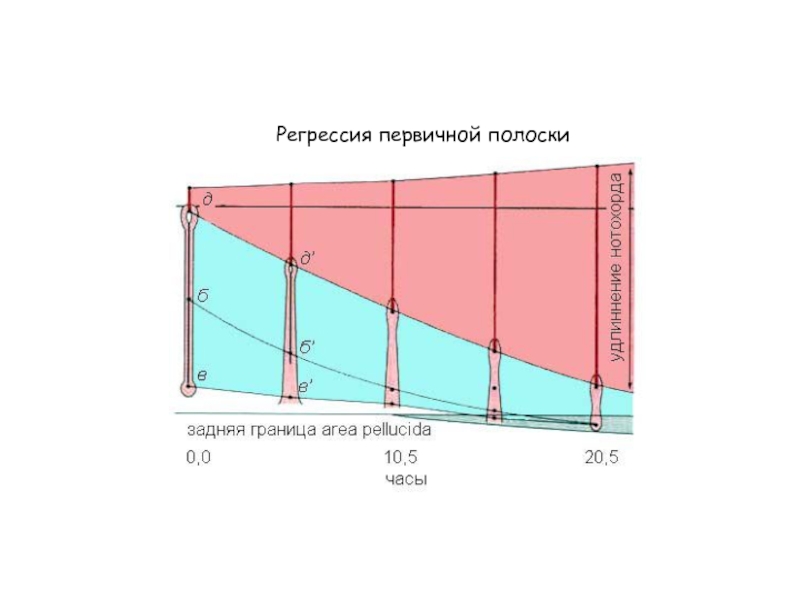
- 31. Регрессия первичной полоски
- 32. Figure 11.15. Formation of Hensen's node from
- 33. FIGURE 8. Whole-mount in situ hybridization of
- 34. Figure 11.14. Regulation of the chick blastoderm.
- 35. Возможный механизм формирования первичной полоски у куриных
- 37. Figure 11.16. Induction of a new embryo
- 38. Figure 1.10. Genetic markers as cell lineage
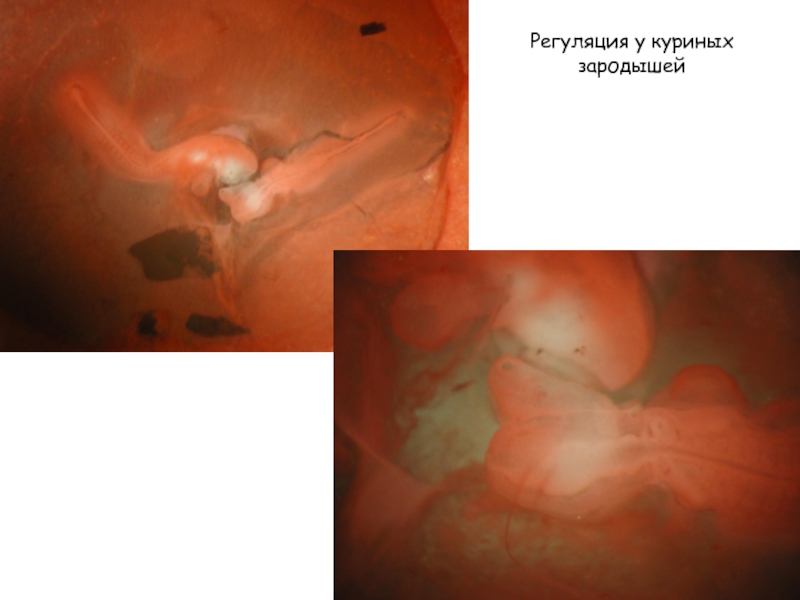
- 39. Регуляция у куриных зародышей
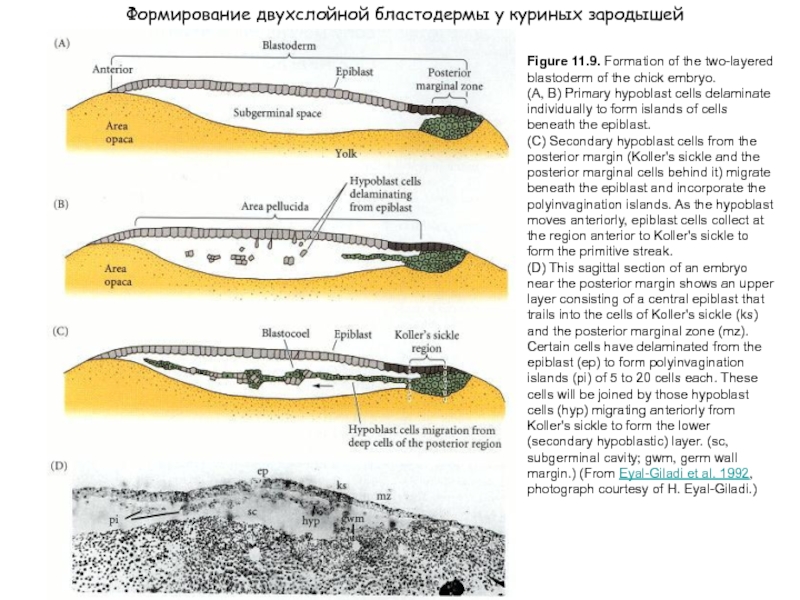
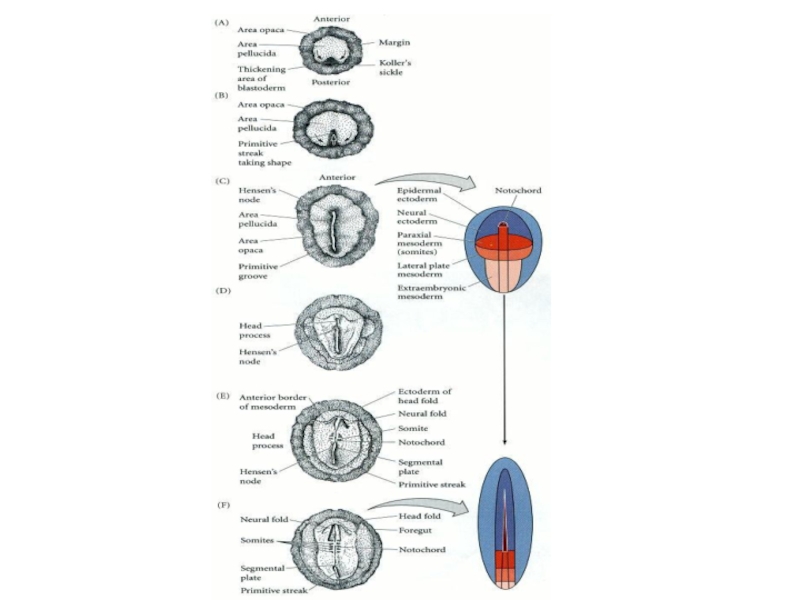
Слайд 3Figure 11.9. Formation of the two-layered blastoderm of the chick embryo.
(A, B) Primary hypoblast cells delaminate individually to form islands of cells beneath the epiblast.
(C) Secondary hypoblast cells from the posterior margin (Koller's sickle and the posterior marginal cells behind it) migrate beneath the epiblast and incorporate the polyinvagination islands. As the hypoblast moves anteriorly, epiblast cells collect at the region anterior to Koller's sickle to form the primitive streak.
(D) This sagittal section of an embryo near the posterior margin shows an upper layer consisting of a central epiblast that trails into the cells of Koller's sickle (ks) and the posterior marginal zone (mz). Certain cells have delaminated from the epiblast (ep) to form polyinvagination islands (pi) of 5 to 20 cells each. These cells will be joined by those hypoblast cells (hyp) migrating anteriorly from Koller's sickle to form the lower (secondary hypoblastic) layer. (sc, subgerminal cavity; gwm, germ wall margin.) (From Eyal-Giladi et al. 1992, photograph courtesy of H. Eyal-Giladi.)
Формирование двухслойной бластодермы у куриных зародышей
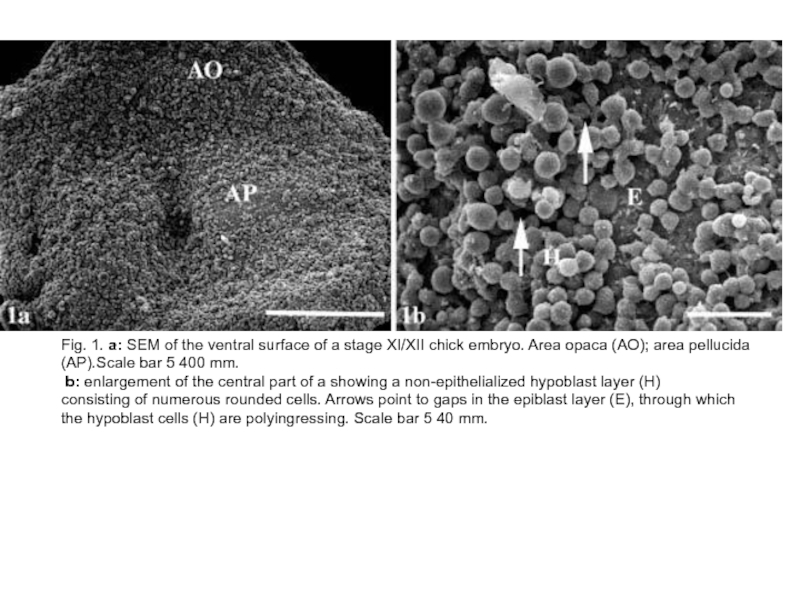
Слайд 4Fig. 1. a: SEM of the ventral surface of a stage
XI/XII chick embryo. Area opaca (AO); area pellucida (AP).Scale bar 5 400 mm.
b: enlargement of the central part of a showing a non-epithelialized hypoblast layer (H)
consisting of numerous rounded cells. Arrows point to gaps in the epiblast layer (E), through which the hypoblast cells (H) are polyingressing. Scale bar 5 40 mm.
b: enlargement of the central part of a showing a non-epithelialized hypoblast layer (H)
consisting of numerous rounded cells. Arrows point to gaps in the epiblast layer (E), through which the hypoblast cells (H) are polyingressing. Scale bar 5 40 mm.
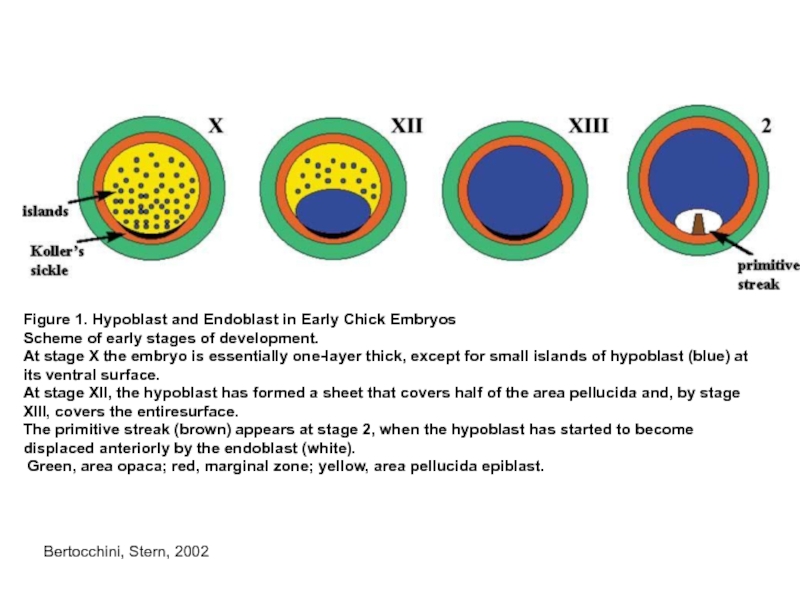
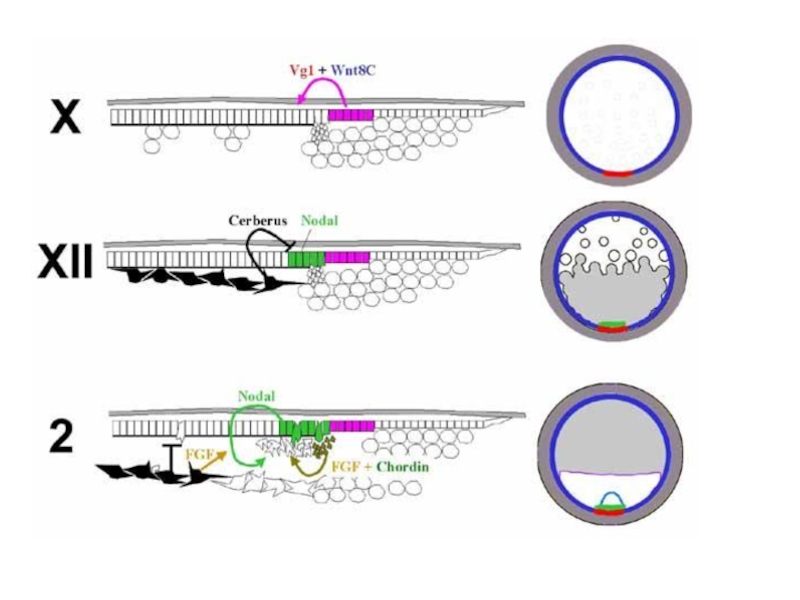
Слайд 5Figure 1. Hypoblast and Endoblast in Early Chick Embryos
Scheme of early
stages of development.
At stage X the embryo is essentially one-layer thick, except for small islands of hypoblast (blue) at
its ventral surface.
At stage XII, the hypoblast has formed a sheet that covers half of the area pellucida and, by stage XIII, covers the entiresurface.
The primitive streak (brown) appears at stage 2, when the hypoblast has started to become displaced anteriorly by the endoblast (white).
Green, area opaca; red, marginal zone; yellow, area pellucida epiblast.
At stage X the embryo is essentially one-layer thick, except for small islands of hypoblast (blue) at
its ventral surface.
At stage XII, the hypoblast has formed a sheet that covers half of the area pellucida and, by stage XIII, covers the entiresurface.
The primitive streak (brown) appears at stage 2, when the hypoblast has started to become displaced anteriorly by the endoblast (white).
Green, area opaca; red, marginal zone; yellow, area pellucida epiblast.
Bertocchini, Stern, 2002
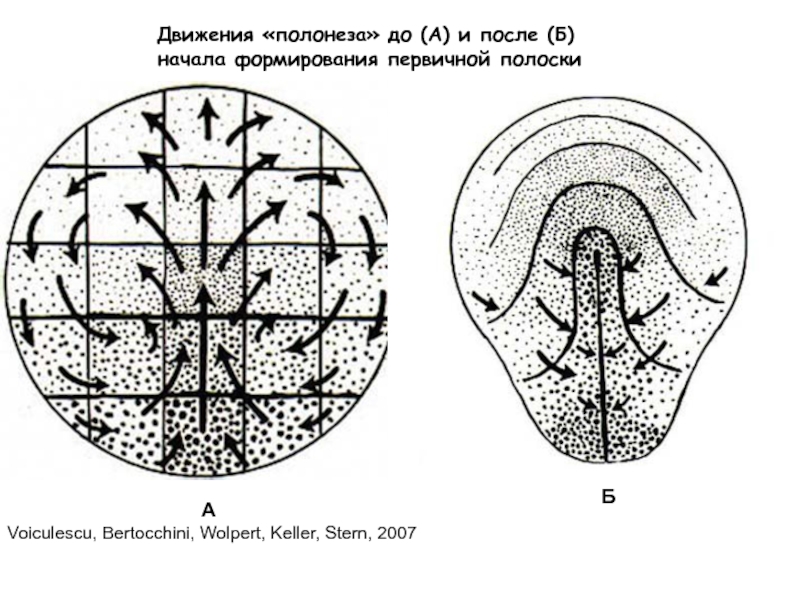
Слайд 7Движения «полонеза» до (А) и после (Б)
начала формирования первичной полоски
А
Б
Voiculescu,
Bertocchini, Wolpert, Keller, Stern, 2007
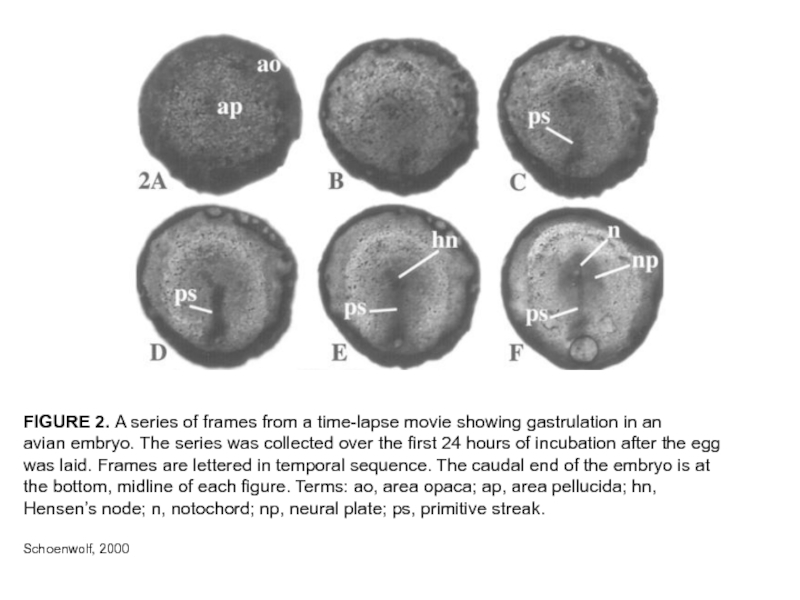
Слайд 9FIGURE 2. A series of frames from a time-lapse movie showing
gastrulation in an
avian embryo. The series was collected over the first 24 hours of incubation after the egg
was laid. Frames are lettered in temporal sequence. The caudal end of the embryo is at the bottom, midline of each figure. Terms: ao, area opaca; ap, area pellucida; hn, Hensen’s node; n, notochord; np, neural plate; ps, primitive streak.
Schoenwolf, 2000
avian embryo. The series was collected over the first 24 hours of incubation after the egg
was laid. Frames are lettered in temporal sequence. The caudal end of the embryo is at the bottom, midline of each figure. Terms: ao, area opaca; ap, area pellucida; hn, Hensen’s node; n, notochord; np, neural plate; ps, primitive streak.
Schoenwolf, 2000
Слайд 11
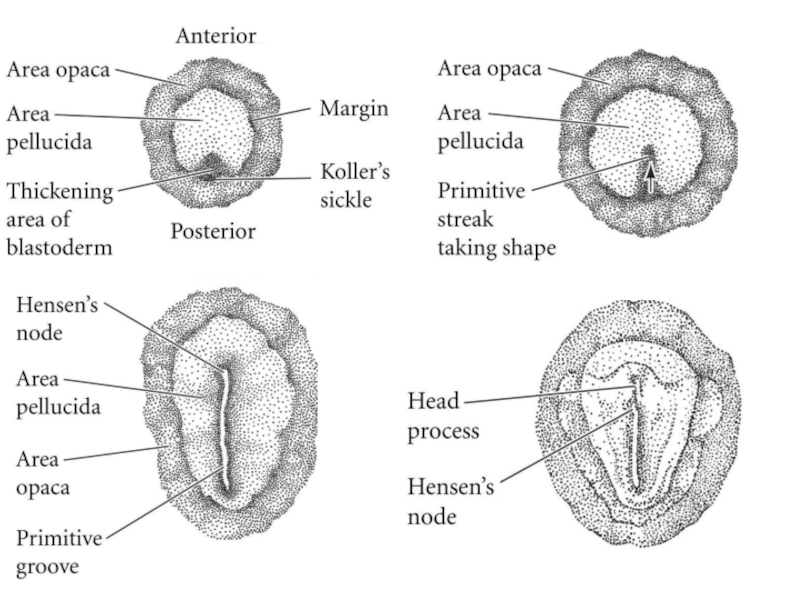
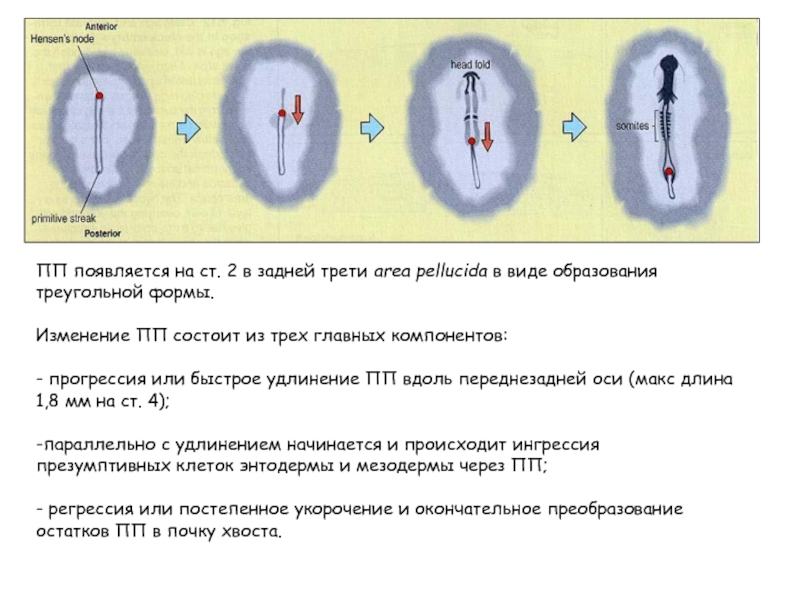
ПП появляется на ст. 2 в задней трети area pellucida в
виде образования треугольной формы.
Изменение ПП состоит из трех главных компонентов:
- прогрессия или быстрое удлинение ПП вдоль переднезадней оси (макс длина 1,8 мм на ст. 4);
-параллельно с удлинением начинается и происходит ингрессия презумптивных клеток энтодермы и мезодермы через ПП;
- регрессия или постепенное укорочение и окончательное преобразование остатков ПП в почку хвоста.
Изменение ПП состоит из трех главных компонентов:
- прогрессия или быстрое удлинение ПП вдоль переднезадней оси (макс длина 1,8 мм на ст. 4);
-параллельно с удлинением начинается и происходит ингрессия презумптивных клеток энтодермы и мезодермы через ПП;
- регрессия или постепенное укорочение и окончательное преобразование остатков ПП в почку хвоста.
Слайд 12FIGURE 3. (A) Scanning electron micrograph of a chick blastoderm in
which the primitive streak (ps) and ingressed mesodermal mantle (m) are viewed en face after removal of the endodermal layer. The embryo is at the stage illustrated in FIGURE 2E. The basal surface of the epiblast is partially visible (ep). The caudal end of the embryo is at the bottom, midline of the figure. The arrow in part A indicates the level of the slice shown in part B. Terms: ao, yolk-ladened cells of the area opaca. (B) Scanning electron micrograph of a transverse slice through the blastoderm at the stage illustrated in FIGURE 2E. The arrows indicate the directions of migration of cells toward, into, and away from the primitive streak (ps). Terms: ep, epiblast; e, endoderm; m, mesoderm. (C) Niagara Falls as shown in a 1956 photograph taken by one of the author’s parents on a family vacation. With the exception of the prospective notochordal cells contained within Hensen’s node, the movement of cells toward, into, and away from the primitive streak occurs in a manner similar to that of water going toward and over the falls, and then flowing downstream. Thus, a constant turnover of cells occurs throughout most of the primitive streak. Schoenwolf, 2000
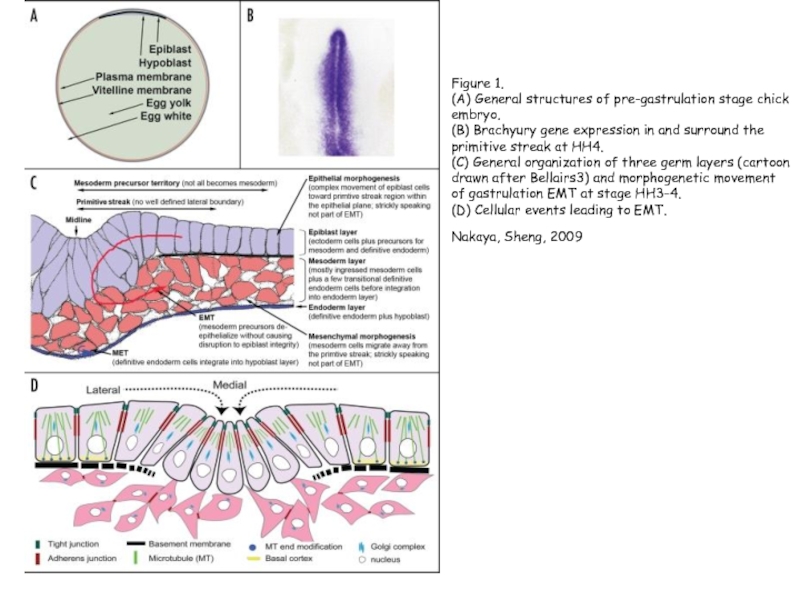
Слайд 14Figure 1.
(A) General structures of pre-gastrulation stage chick embryo.
(B)
Brachyury gene expression in and surround the primitive streak at HH4.
(C) General organization of three germ layers (cartoon drawn after Bellairs3) and morphogenetic movement of gastrulation EMT at stage HH3–4.
(D) Cellular events leading to EMT.
Nakaya, Sheng, 2009
(C) General organization of three germ layers (cartoon drawn after Bellairs3) and morphogenetic movement of gastrulation EMT at stage HH3–4.
(D) Cellular events leading to EMT.
Nakaya, Sheng, 2009
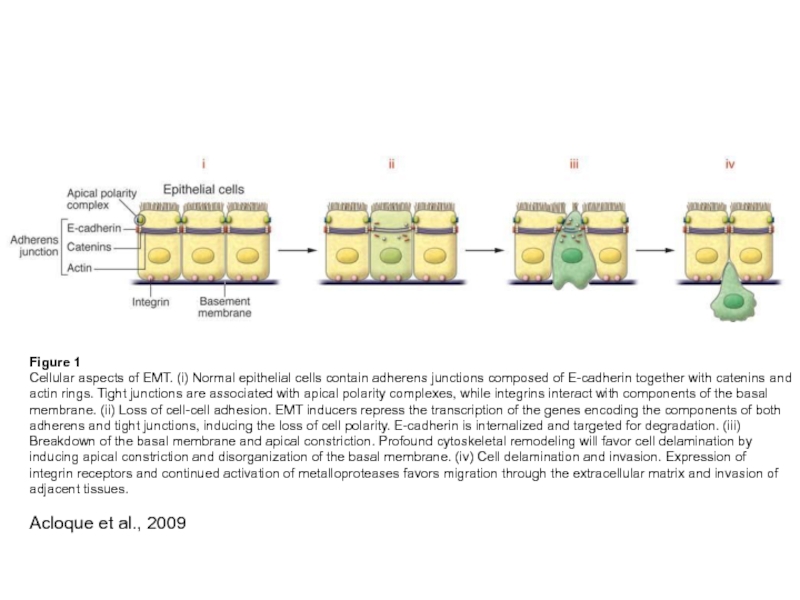
Слайд 16Figure 1
Cellular aspects of EMT. (i) Normal epithelial cells contain adherens
junctions composed of E-cadherin together with catenins and actin rings. Tight junctions are associated with apical polarity complexes, while integrins interact with components of the basal membrane. (ii) Loss of cell-cell adhesion. EMT inducers repress the transcription of the genes encoding the components of both adherens and tight junctions, inducing the loss of cell polarity. E-cadherin is internalized and targeted for degradation. (iii) Breakdown of the basal membrane and apical constriction. Profound cytoskeletal remodeling will favor cell delamination by inducing apical constriction and disorganization of the basal membrane. (iv) Cell delamination and invasion. Expression of integrin receptors and continued activation of metalloproteases favors migration through the extracellular matrix and invasion of adjacent tissues.
Acloque et al., 2009
Acloque et al., 2009
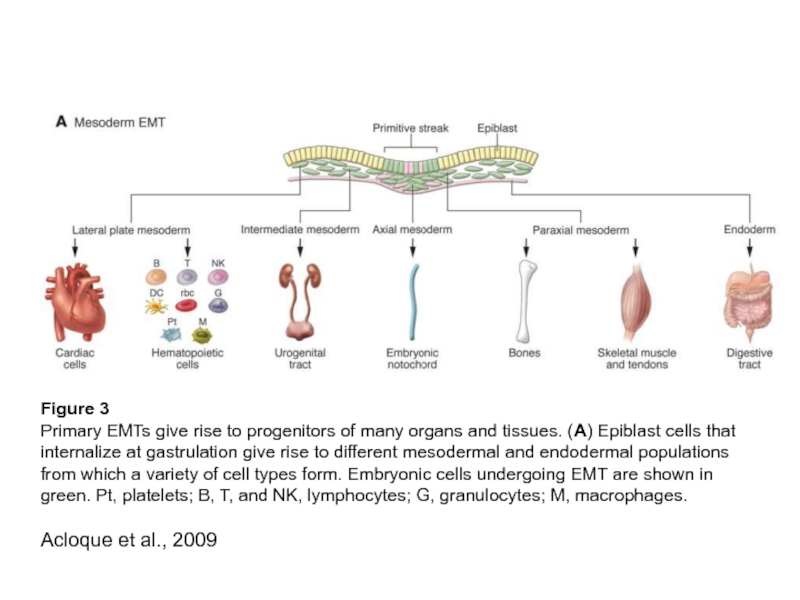
Слайд 17Figure 3
Primary EMTs give rise to progenitors of many organs and
tissues. (A) Epiblast cells that internalize at gastrulation give rise to different mesodermal and endodermal populations from which a variety of cell types form. Embryonic cells undergoing EMT are shown in green. Pt, platelets; B, T, and NK, lymphocytes; G, granulocytes; M, macrophages.
Acloque et al., 2009
Acloque et al., 2009
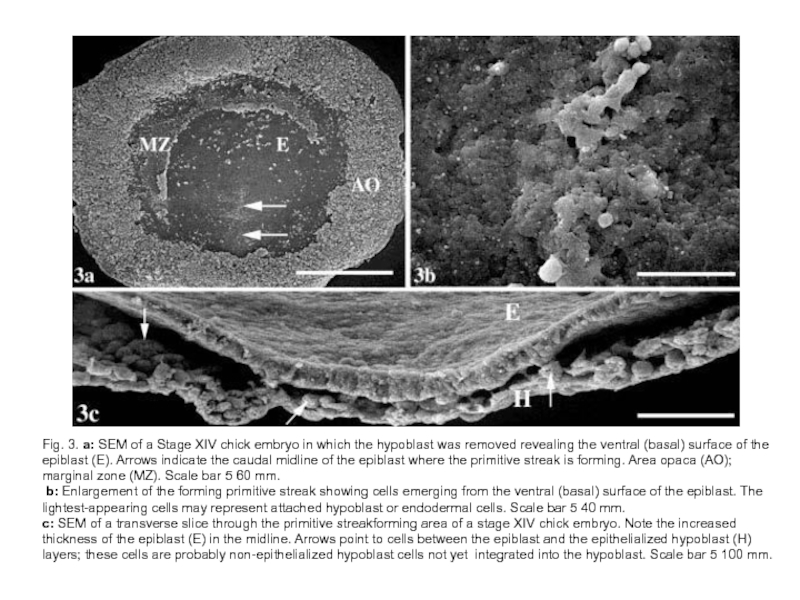
Слайд 18Fig. 3. a: SEM of a Stage XIV chick embryo in
which the hypoblast was removed revealing the ventral (basal) surface of the epiblast (E). Arrows indicate the caudal midline of the epiblast where the primitive streak is forming. Area opaca (AO); marginal zone (MZ). Scale bar 5 60 mm.
b: Enlargement of the forming primitive streak showing cells emerging from the ventral (basal) surface of the epiblast. The lightest-appearing cells may represent attached hypoblast or endodermal cells. Scale bar 5 40 mm.
c: SEM of a transverse slice through the primitive streakforming area of a stage XIV chick embryo. Note the increased thickness of the epiblast (E) in the midline. Arrows point to cells between the epiblast and the epithelialized hypoblast (H) layers; these cells are probably non-epithelialized hypoblast cells not yet integrated into the hypoblast. Scale bar 5 100 mm.
b: Enlargement of the forming primitive streak showing cells emerging from the ventral (basal) surface of the epiblast. The lightest-appearing cells may represent attached hypoblast or endodermal cells. Scale bar 5 40 mm.
c: SEM of a transverse slice through the primitive streakforming area of a stage XIV chick embryo. Note the increased thickness of the epiblast (E) in the midline. Arrows point to cells between the epiblast and the epithelialized hypoblast (H) layers; these cells are probably non-epithelialized hypoblast cells not yet integrated into the hypoblast. Scale bar 5 100 mm.
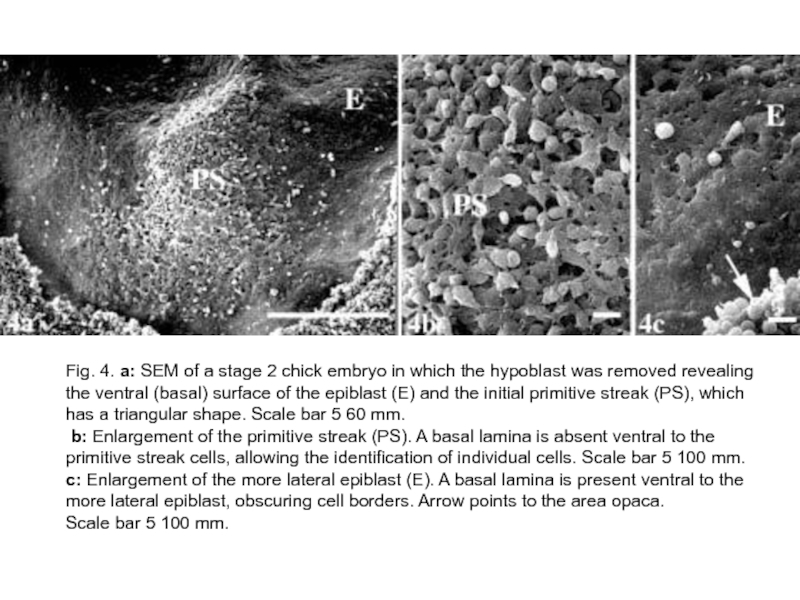
Слайд 19Fig. 4. a: SEM of a stage 2 chick embryo in
which the hypoblast was removed revealing the ventral (basal) surface of the epiblast (E) and the initial primitive streak (PS), which has a triangular shape. Scale bar 5 60 mm.
b: Enlargement of the primitive streak (PS). A basal lamina is absent ventral to the primitive streak cells, allowing the identification of individual cells. Scale bar 5 100 mm. c: Enlargement of the more lateral epiblast (E). A basal lamina is present ventral to the more lateral epiblast, obscuring cell borders. Arrow points to the area opaca.
Scale bar 5 100 mm.
b: Enlargement of the primitive streak (PS). A basal lamina is absent ventral to the primitive streak cells, allowing the identification of individual cells. Scale bar 5 100 mm. c: Enlargement of the more lateral epiblast (E). A basal lamina is present ventral to the more lateral epiblast, obscuring cell borders. Arrow points to the area opaca.
Scale bar 5 100 mm.
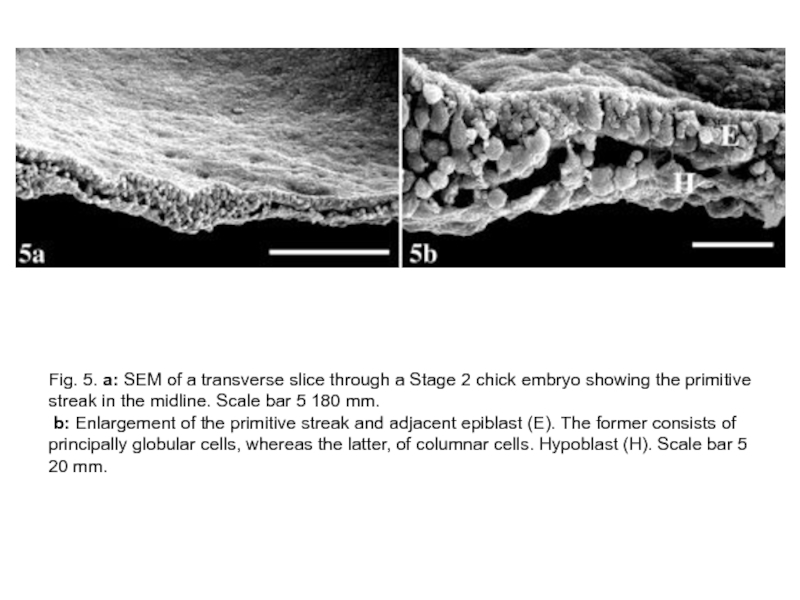
Слайд 20Fig. 5. a: SEM of a transverse slice through a Stage
2 chick embryo showing the primitive streak in the midline. Scale bar 5 180 mm.
b: Enlargement of the primitive streak and adjacent epiblast (E). The former consists of principally globular cells, whereas the latter, of columnar cells. Hypoblast (H). Scale bar 5 20 mm.
b: Enlargement of the primitive streak and adjacent epiblast (E). The former consists of principally globular cells, whereas the latter, of columnar cells. Hypoblast (H). Scale bar 5 20 mm.
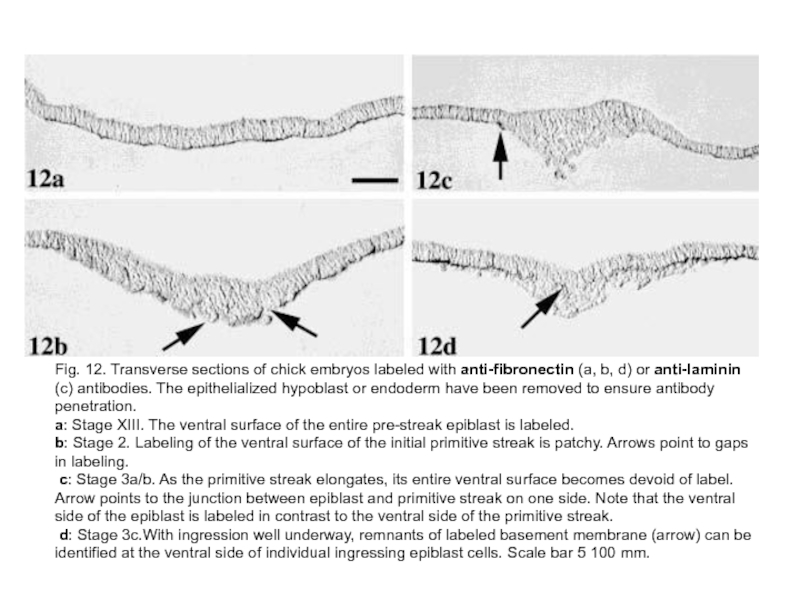
Слайд 21Fig. 12. Transverse sections of chick embryos labeled with anti-fibronectin (a,
b, d) or anti-laminin (c) antibodies. The epithelialized hypoblast or endoderm have been removed to ensure antibody penetration.
a: Stage XIII. The ventral surface of the entire pre-streak epiblast is labeled.
b: Stage 2. Labeling of the ventral surface of the initial primitive streak is patchy. Arrows point to gaps in labeling.
c: Stage 3a/b. As the primitive streak elongates, its entire ventral surface becomes devoid of label. Arrow points to the junction between epiblast and primitive streak on one side. Note that the ventral side of the epiblast is labeled in contrast to the ventral side of the primitive streak.
d: Stage 3c.With ingression well underway, remnants of labeled basement membrane (arrow) can be identified at the ventral side of individual ingressing epiblast cells. Scale bar 5 100 mm.
a: Stage XIII. The ventral surface of the entire pre-streak epiblast is labeled.
b: Stage 2. Labeling of the ventral surface of the initial primitive streak is patchy. Arrows point to gaps in labeling.
c: Stage 3a/b. As the primitive streak elongates, its entire ventral surface becomes devoid of label. Arrow points to the junction between epiblast and primitive streak on one side. Note that the ventral side of the epiblast is labeled in contrast to the ventral side of the primitive streak.
d: Stage 3c.With ingression well underway, remnants of labeled basement membrane (arrow) can be identified at the ventral side of individual ingressing epiblast cells. Scale bar 5 100 mm.
Слайд 22Механизмы удлинения ПП :
конвергенция-растяжение материала ПП, увеличение длины коррелирует с
уменьшением ширины ПП
(дл 770-1475 мкм, шир 325-145 мкм);
ингрессия клеток из Э на ростральном конце ПП;
- ориентированные клеточные деления, когда большая часть веретен делений ориентирована вдоль передне-задней оси.
(дл 770-1475 мкм, шир 325-145 мкм);
ингрессия клеток из Э на ростральном конце ПП;
- ориентированные клеточные деления, когда большая часть веретен делений ориентирована вдоль передне-задней оси.
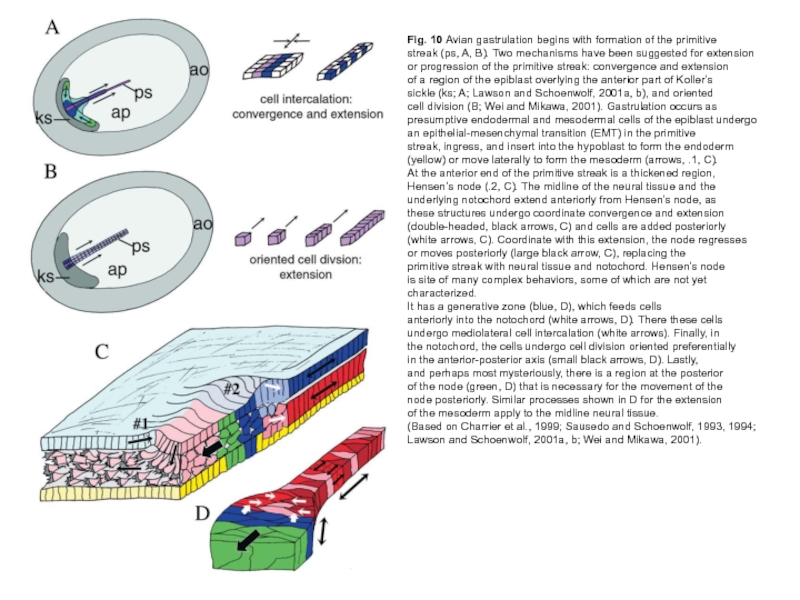
Слайд 23Fig. 10 Avian gastrulation begins with formation of the primitive
streak (ps,
A, B). Two mechanisms have been suggested for extension
or progression of the primitive streak: convergence and extension
of a region of the epiblast overlying the anterior part of Koller’s
sickle (ks; A; Lawson and Schoenwolf, 2001a, b), and oriented
cell division (B; Wei and Mikawa, 2001). Gastrulation occurs as
presumptive endodermal and mesodermal cells of the epiblast undergo
an epithelial-mesenchymal transition (EMT) in the primitive
streak, ingress, and insert into the hypoblast to form the endoderm
(yellow) or move laterally to form the mesoderm (arrows, .1, C).
At the anterior end of the primitive streak is a thickened region,
Hensen’s node (.2, C). The midline of the neural tissue and the
underlying notochord extend anteriorly from Hensen’s node, as
these structures undergo coordinate convergence and extension
(double-headed, black arrows, C) and cells are added posteriorly
(white arrows, C). Coordinate with this extension, the node regresses
or moves posteriorly (large black arrow, C), replacing the
primitive streak with neural tissue and notochord. Hensen’s node
is site of many complex behaviors, some of which are not yet characterized.
It has a generative zone (blue, D), which feeds cells
anteriorly into the notochord (white arrows, D). There these cells
undergo mediolateral cell intercalation (white arrows). Finally, in
the notochord, the cells undergo cell division oriented preferentially
in the anterior-posterior axis (small black arrows, D). Lastly,
and perhaps most mysteriously, there is a region at the posterior
of the node (green, D) that is necessary for the movement of the
node posteriorly. Similar processes shown in D for the extension
of the mesoderm apply to the midline neural tissue.
(Based on Charrier et al., 1999; Sausedo and Schoenwolf, 1993, 1994; Lawson and Schoenwolf, 2001a, b; Wei and Mikawa, 2001).
or progression of the primitive streak: convergence and extension
of a region of the epiblast overlying the anterior part of Koller’s
sickle (ks; A; Lawson and Schoenwolf, 2001a, b), and oriented
cell division (B; Wei and Mikawa, 2001). Gastrulation occurs as
presumptive endodermal and mesodermal cells of the epiblast undergo
an epithelial-mesenchymal transition (EMT) in the primitive
streak, ingress, and insert into the hypoblast to form the endoderm
(yellow) or move laterally to form the mesoderm (arrows, .1, C).
At the anterior end of the primitive streak is a thickened region,
Hensen’s node (.2, C). The midline of the neural tissue and the
underlying notochord extend anteriorly from Hensen’s node, as
these structures undergo coordinate convergence and extension
(double-headed, black arrows, C) and cells are added posteriorly
(white arrows, C). Coordinate with this extension, the node regresses
or moves posteriorly (large black arrow, C), replacing the
primitive streak with neural tissue and notochord. Hensen’s node
is site of many complex behaviors, some of which are not yet characterized.
It has a generative zone (blue, D), which feeds cells
anteriorly into the notochord (white arrows, D). There these cells
undergo mediolateral cell intercalation (white arrows). Finally, in
the notochord, the cells undergo cell division oriented preferentially
in the anterior-posterior axis (small black arrows, D). Lastly,
and perhaps most mysteriously, there is a region at the posterior
of the node (green, D) that is necessary for the movement of the
node posteriorly. Similar processes shown in D for the extension
of the mesoderm apply to the midline neural tissue.
(Based on Charrier et al., 1999; Sausedo and Schoenwolf, 1993, 1994; Lawson and Schoenwolf, 2001a, b; Wei and Mikawa, 2001).
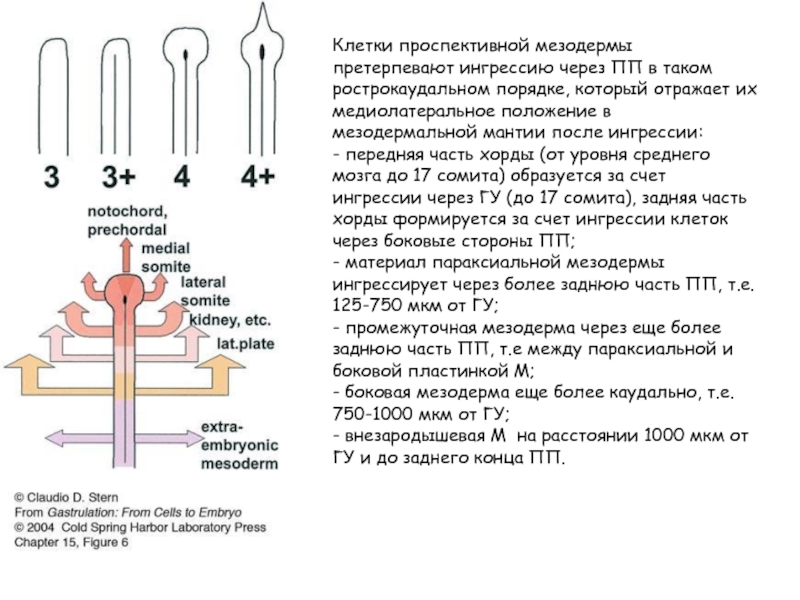
Слайд 25Клетки проспективной мезодермы претерпевают ингрессию через ПП в таком рострокаудальном порядке,
который отражает их медиолатеральное положение в мезодермальной мантии после ингрессии:
- передняя часть хорды (от уровня среднего мозга до 17 сомита) образуется за счет ингрессии через ГУ (до 17 сомита), задняя часть хорды формируется за счет ингрессии клеток через боковые стороны ПП;
- материал параксиальной мезодермы ингрессирует через более заднюю часть ПП, т.е. 125-750 мкм от ГУ;
- промежуточная мезодерма через еще более заднюю часть ПП, т.е между параксиальной и боковой пластинкой М;
- боковая мезодерма еще более каудально, т.е. 750-1000 мкм от ГУ;
- внезародышевая М на расстоянии 1000 мкм от ГУ и до заднего конца ПП.
- передняя часть хорды (от уровня среднего мозга до 17 сомита) образуется за счет ингрессии через ГУ (до 17 сомита), задняя часть хорды формируется за счет ингрессии клеток через боковые стороны ПП;
- материал параксиальной мезодермы ингрессирует через более заднюю часть ПП, т.е. 125-750 мкм от ГУ;
- промежуточная мезодерма через еще более заднюю часть ПП, т.е между параксиальной и боковой пластинкой М;
- боковая мезодерма еще более каудально, т.е. 750-1000 мкм от ГУ;
- внезародышевая М на расстоянии 1000 мкм от ГУ и до заднего конца ПП.
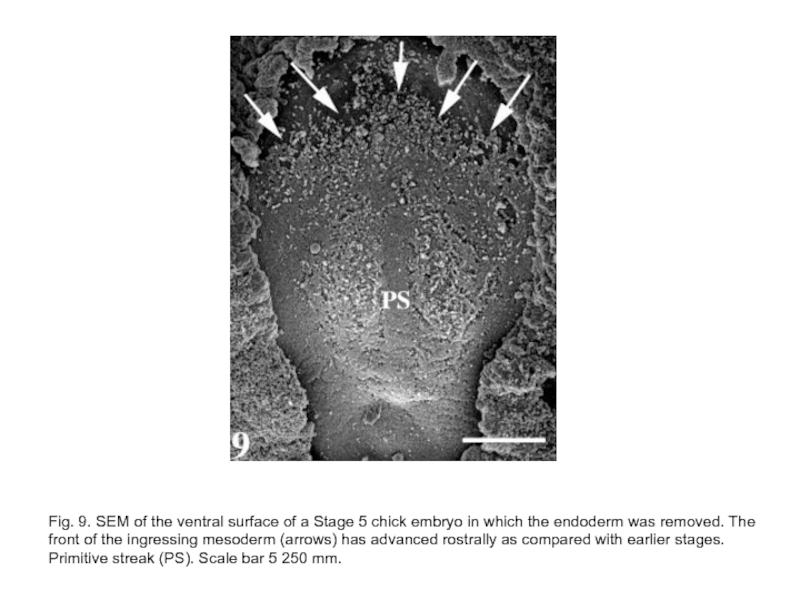
Слайд 26Fig. 9. SEM of the ventral surface of a Stage 5
chick embryo in which the endoderm was removed. The front of the ingressing mesoderm (arrows) has advanced rostrally as compared with earlier stages. Primitive streak (PS). Scale bar 5 250 mm.
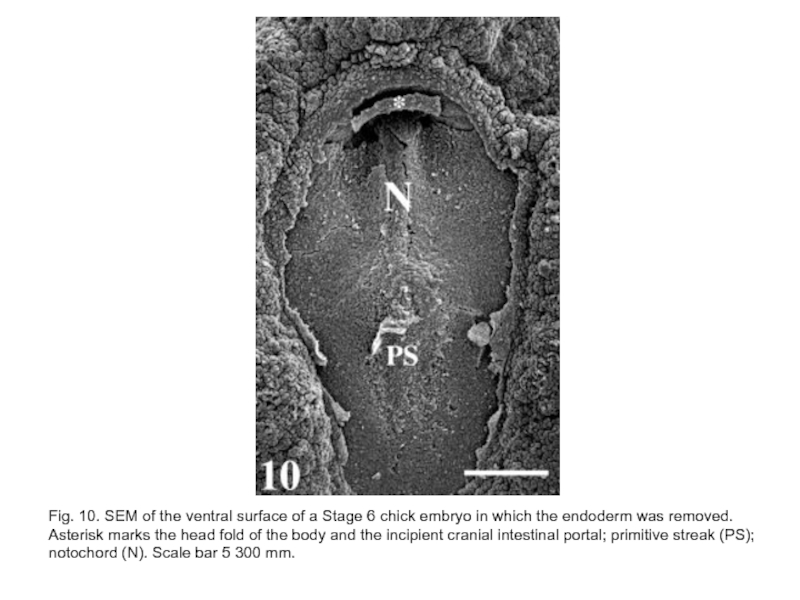
Слайд 27Fig. 10. SEM of the ventral surface of a Stage 6
chick embryo in which the endoderm was removed. Asterisk marks the head fold of the body and the incipient cranial intestinal portal; primitive streak (PS);
notochord (N). Scale bar 5 300 mm.
notochord (N). Scale bar 5 300 mm.
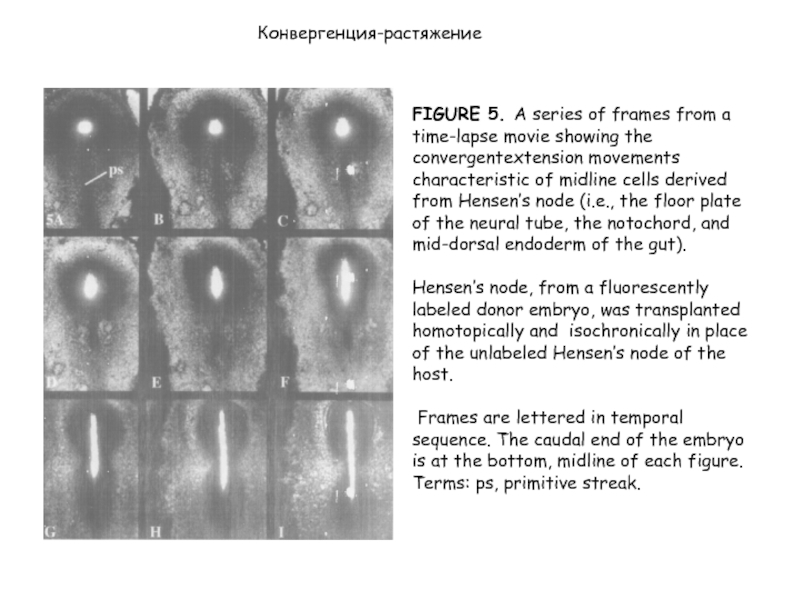
Слайд 28FIGURE 5. A series of frames from a time-lapse movie showing
the сonvergentextension movements characteristic of midline cells derived from Hensen’s node (i.e., the floor plate of the neural tube, the notochord, and mid-dorsal endoderm of the gut).
Hensen’s node, from a fluorescently labeled donor embryo, was transplanted homotopically and isochronically in place of the unlabeled Hensen’s node of the host.
Frames are lettered in temporal sequence. The caudal end of the embryo is at the bottom, midline of each figure.
Terms: ps, primitive streak.
Hensen’s node, from a fluorescently labeled donor embryo, was transplanted homotopically and isochronically in place of the unlabeled Hensen’s node of the host.
Frames are lettered in temporal sequence. The caudal end of the embryo is at the bottom, midline of each figure.
Terms: ps, primitive streak.
Конвергенция-растяжение
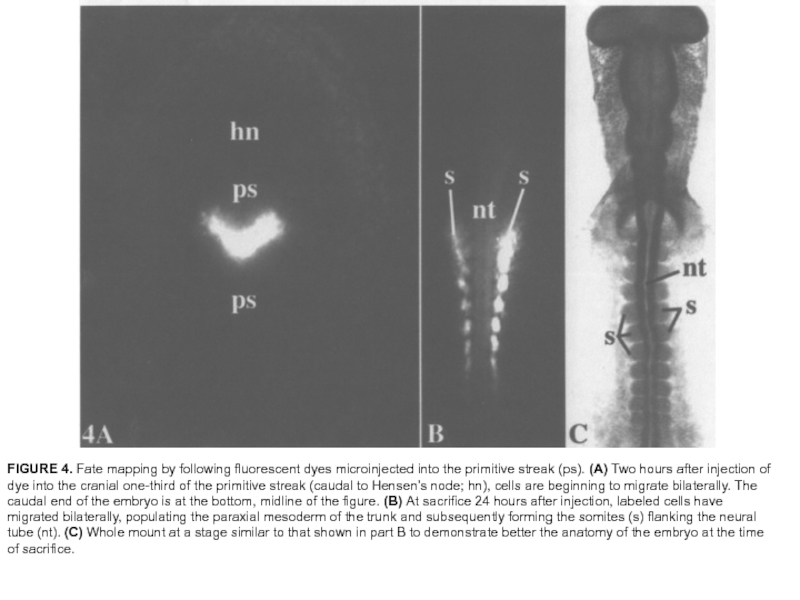
Слайд 29FIGURE 4. Fate mapping by following fluorescent dyes microinjected into the
primitive streak (ps). (A) Two hours after injection of dye into the cranial one-third of the primitive streak (caudal to Hensen’s node; hn), cells are beginning to migrate bilaterally. The caudal end of the embryo is at the bottom, midline of the figure. (B) At sacrifice 24 hours after injection, labeled cells have migrated bilaterally, populating the paraxial mesoderm of the trunk and subsequently forming the somites (s) flanking the neural tube (nt). (C) Whole mount at a stage similar to that shown in part B to demonstrate better the anatomy of the embryo at the time of sacrifice.
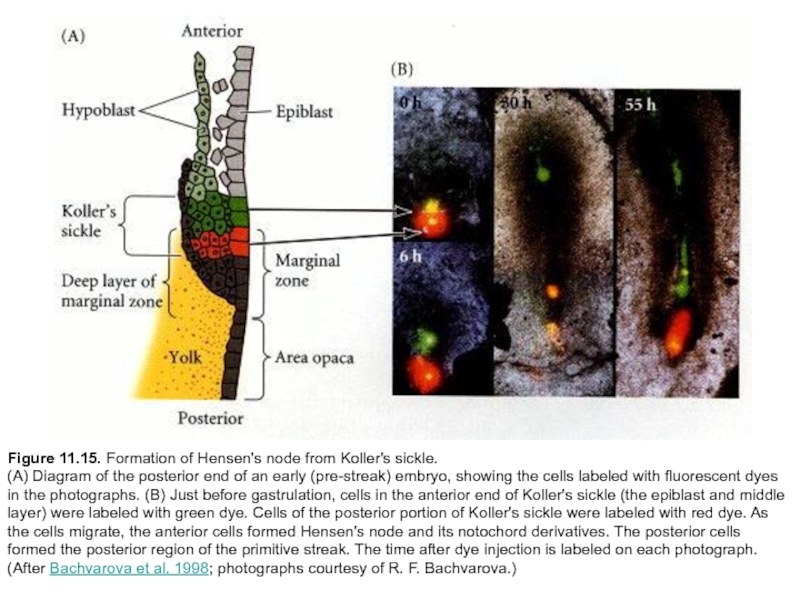
Слайд 32Figure 11.15. Formation of Hensen's node from Koller's sickle.
(A) Diagram
of the posterior end of an early (pre-streak) embryo, showing the cells labeled with fluorescent dyes in the photographs. (B) Just before gastrulation, cells in the anterior end of Koller's sickle (the epiblast and middle layer) were labeled with green dye. Cells of the posterior portion of Koller's sickle were labeled with red dye. As the cells migrate, the anterior cells formed Hensen's node and its notochord derivatives. The posterior cells formed the posterior region of the primitive streak. The time after dye injection is labeled on each photograph. (After Bachvarova et al. 1998; photographs courtesy of R. F. Bachvarova.)
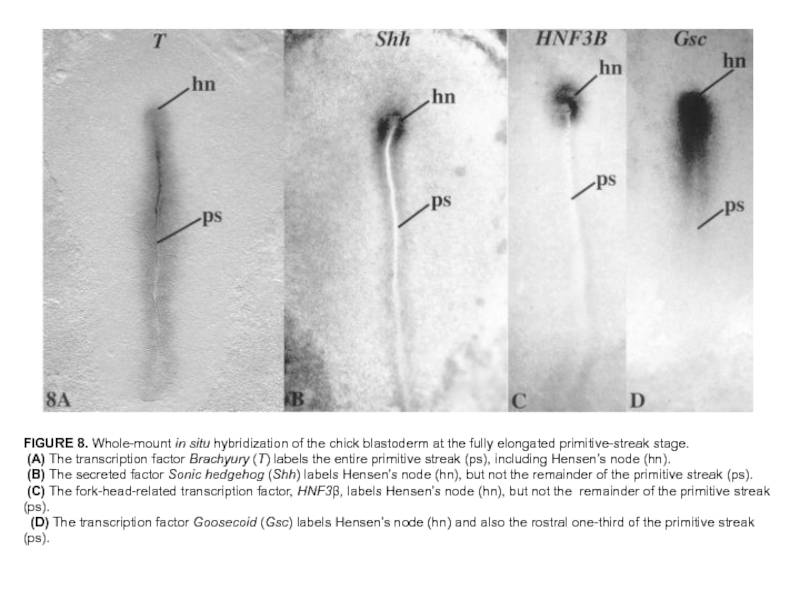
Слайд 33FIGURE 8. Whole-mount in situ hybridization of the chick blastoderm at
the fully elongated primitive-streak stage.
(A) The transcription factor Brachyury (T) labels the entire primitive streak (ps), including Hensen’s node (hn).
(B) The secreted factor Sonic hedgehog (Shh) labels Hensen’s node (hn), but not the remainder of the primitive streak (ps).
(C) The fork-head-related transcription factor, HNF3β, labels Hensen’s node (hn), but not the remainder of the primitive streak (ps).
(D) The transcription factor Goosecoid (Gsc) labels Hensen’s node (hn) and also the rostral one-third of the primitive streak (ps).
(A) The transcription factor Brachyury (T) labels the entire primitive streak (ps), including Hensen’s node (hn).
(B) The secreted factor Sonic hedgehog (Shh) labels Hensen’s node (hn), but not the remainder of the primitive streak (ps).
(C) The fork-head-related transcription factor, HNF3β, labels Hensen’s node (hn), but not the remainder of the primitive streak (ps).
(D) The transcription factor Goosecoid (Gsc) labels Hensen’s node (hn) and also the rostral one-third of the primitive streak (ps).
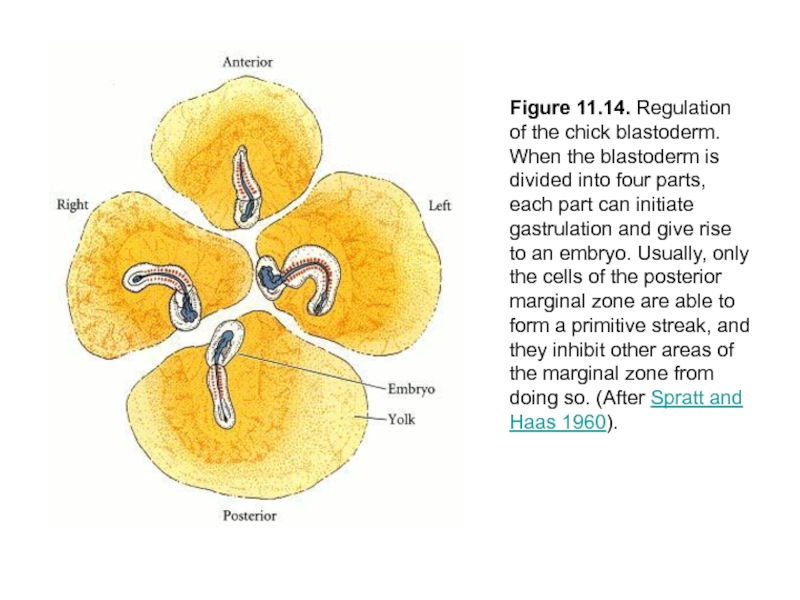
Слайд 34Figure 11.14. Regulation of the chick blastoderm. When the blastoderm is
divided into four parts, each part can initiate gastrulation and give rise to an embryo. Usually, only the cells of the posterior marginal zone are able to form a primitive streak, and they inhibit other areas of the marginal zone from doing so. (After Spratt and Haas 1960).
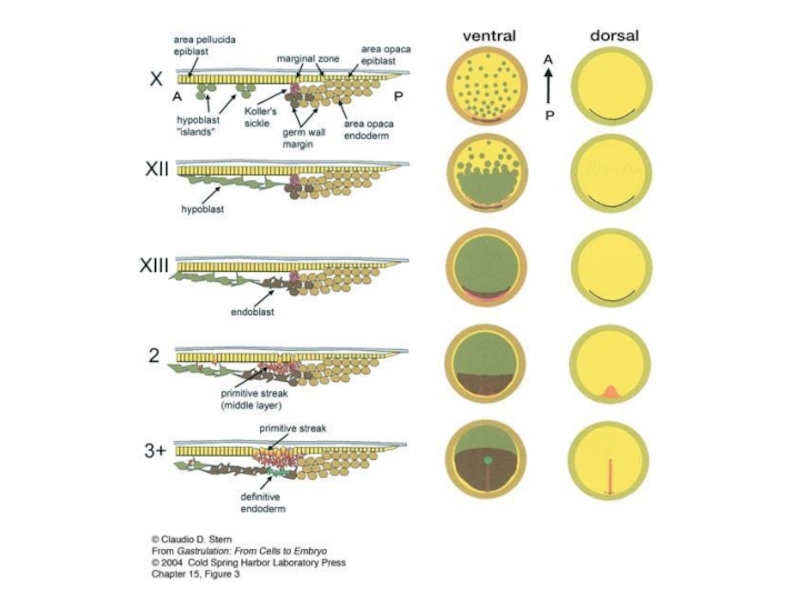
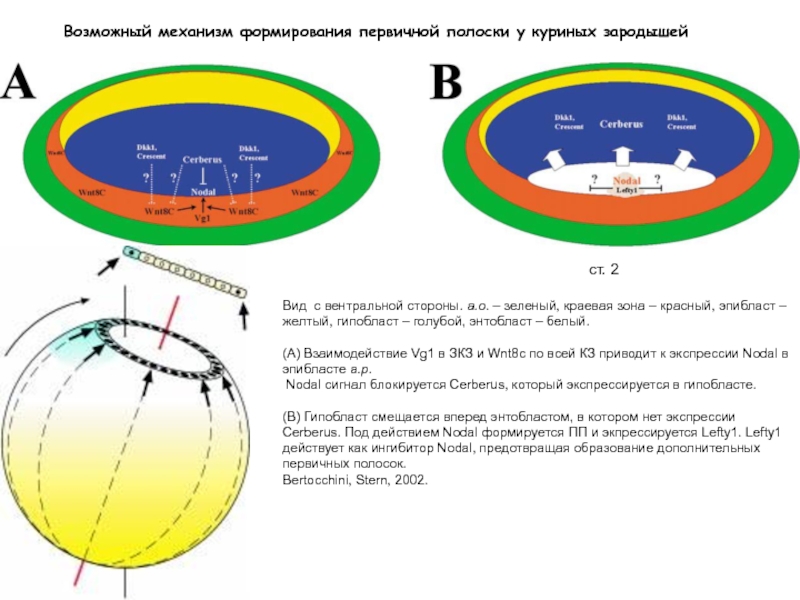
Слайд 35Возможный механизм формирования первичной полоски у куриных зародышей
cт. XII
ст. 2
Вид с
вентральной стороны. a.o. – зеленый, краевая зона – красный, эпибласт – желтый, гипобласт – голубой, энтобласт – белый.
(А) Взаимодействие Vg1 в ЗКЗ и Wnt8c по всей КЗ приводит к экспрессии Nodal в эпибласте a.p.
Nodal сигнал блокируется Cerberus, который экспрессируется в гипобласте.
(В) Гипобласт смещается вперед энтобластом, в котором нет экспрессии Cerberus. Под действием Nodal формируется ПП и экпрессируется Lefty1. Lefty1 действует как ингибитор Nodal, предотвращая образование дополнительных первичных полосок.
Bertocchini, Stern, 2002.
(А) Взаимодействие Vg1 в ЗКЗ и Wnt8c по всей КЗ приводит к экспрессии Nodal в эпибласте a.p.
Nodal сигнал блокируется Cerberus, который экспрессируется в гипобласте.
(В) Гипобласт смещается вперед энтобластом, в котором нет экспрессии Cerberus. Под действием Nodal формируется ПП и экпрессируется Lefty1. Lefty1 действует как ингибитор Nodal, предотвращая образование дополнительных первичных полосок.
Bertocchini, Stern, 2002.
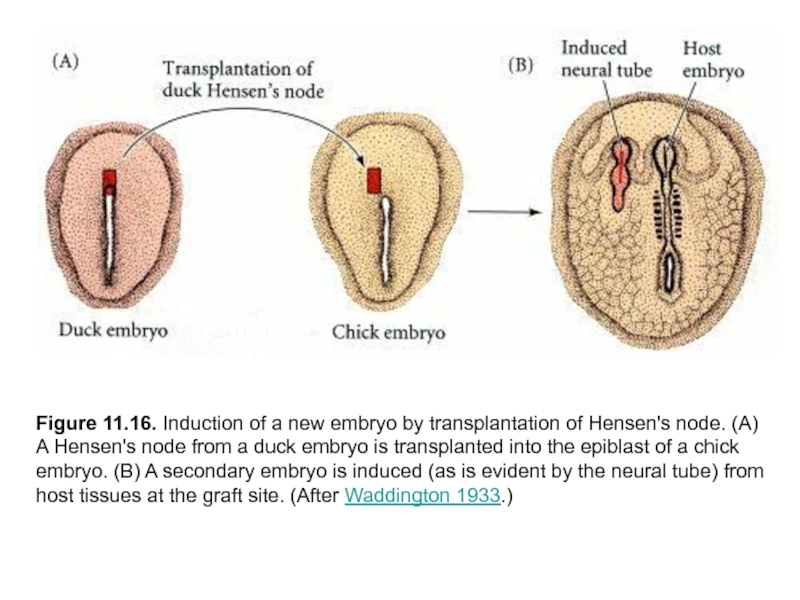
Слайд 37Figure 11.16. Induction of a new embryo by transplantation of Hensen's
node. (A) A Hensen's node from a duck embryo is transplanted into the epiblast of a chick embryo. (B) A secondary embryo is induced (as is evident by the neural tube) from host tissues at the graft site. (After Waddington 1933.)
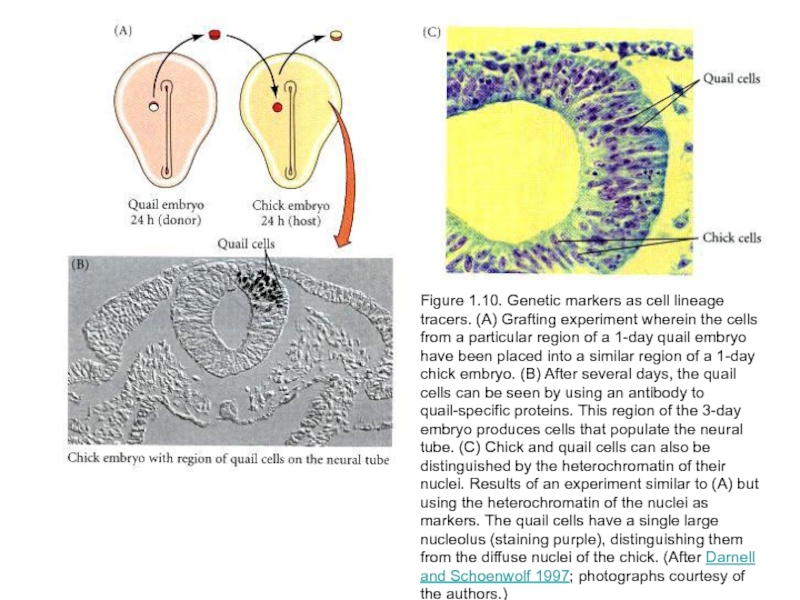
Слайд 38Figure 1.10. Genetic markers as cell lineage tracers. (A) Grafting experiment
wherein the cells from a particular region of a 1-day quail embryo have been placed into a similar region of a 1-day chick embryo. (B) After several days, the quail cells can be seen by using an antibody to quail-specific proteins. This region of the 3-day embryo produces cells that populate the neural tube. (C) Chick and quail cells can also be distinguished by the heterochromatin of their nuclei. Results of an experiment similar to (A) but using the heterochromatin of the nuclei as markers. The quail cells have a single large nucleolus (staining purple), distinguishing them from the diffuse nuclei of the chick. (After Darnell and Schoenwolf 1997; photographs courtesy of the authors.)