Yulia Vitalievna
2016
- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Acid-base equilibrium in biological systems презентация
Содержание
- 1. Acid-base equilibrium in biological systems
- 2. Electrolytes are the substances which
- 3. Classification of electrolytes:
- 4. Protolytic equilibrium in water The ionization of
- 5. Hydrogen ion exponent In pure liquid water
- 6. Hydrogen ions have catalytic effect in many
- 7. BUFFER SOLUTIONS are the solutions which pH
- 8. Buffer systems of blood are the most
- 9. ACIDOSIS and ALKALOSIS Gaseous (respiratory) Non-gaseous:
Слайд 1 Zaporizhzhya State Medical University Analytical Chemistry Department Acid-base equilibrium in biological systems Lecturer: Monaykina
Слайд 2
Electrolytes
are the substances which solutions conduct electric current.
In 1884 S. Arrhenius founded a comprehensive theory which is known as
theory of electrolytic dissociation or ionic theory.
theory of electrolytic dissociation or ionic theory.
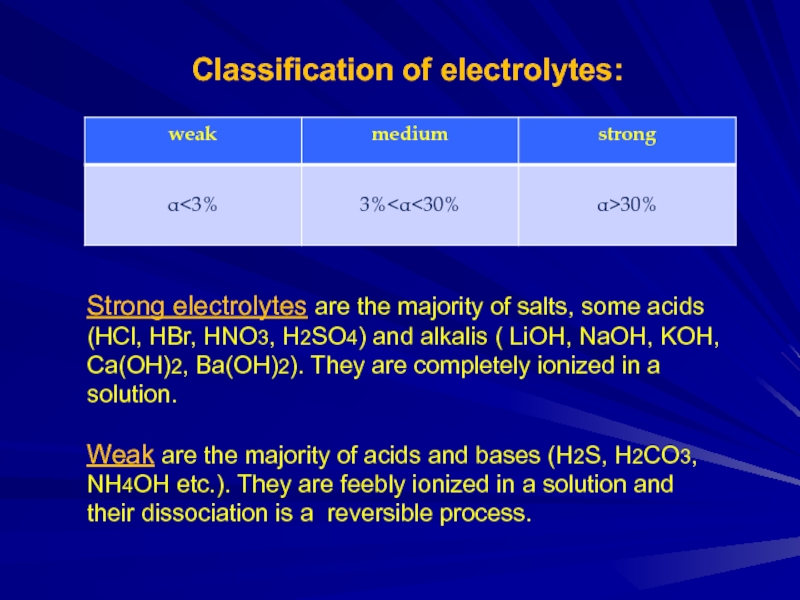
Слайд 3Classification of electrolytes:
Strong electrolytes are the majority of salts, some acids
(HCl, HBr, HNO3, H2SO4) and alkalis ( LiOH, NaOH, KOH, Ca(OH)2, Ba(OH)2). They are completely ionized in a solution.
Weak are the majority of acids and bases (H2S, H2CO3, NH4OH etc.). They are feebly ionized in a solution and their dissociation is a reversible process.
Weak are the majority of acids and bases (H2S, H2CO3, NH4OH etc.). They are feebly ionized in a solution and their dissociation is a reversible process.
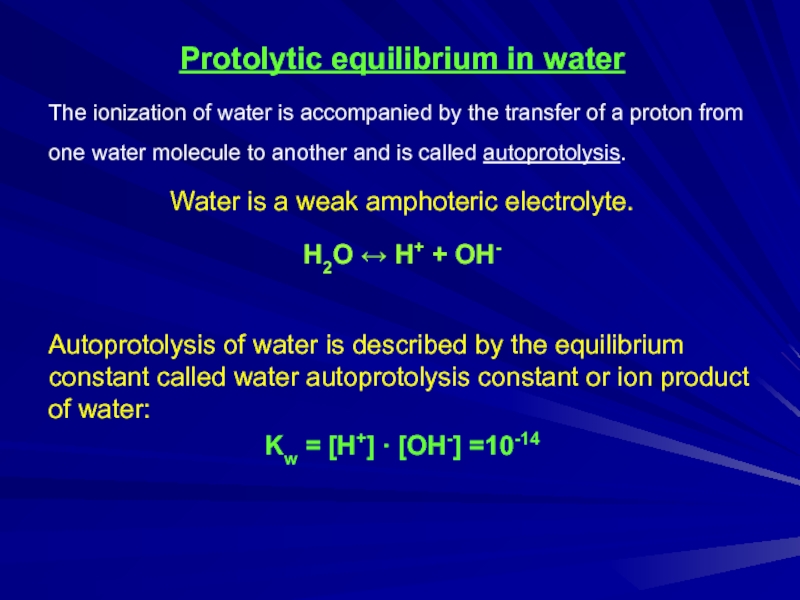
Слайд 4Protolytic equilibrium in water
The ionization of water is accompanied by the
transfer of a proton from one water molecule to another and is called autoprotolysis.
Water is a weak amphoteric electrolyte.
H2О ↔ H+ + OH-
Autoprotolysis of water is described by the equilibrium constant called water autoprotolysis constant or ion product of water:
Kw = [H+] ∙ [OH-] =10-14
Water is a weak amphoteric electrolyte.
H2О ↔ H+ + OH-
Autoprotolysis of water is described by the equilibrium constant called water autoprotolysis constant or ion product of water:
Kw = [H+] ∙ [OH-] =10-14
Слайд 5Hydrogen ion exponent
In pure liquid water (neutral medium) concentrations of hydrogen
and hydroxyl ions are the same:
[H+] = [OH-] = 10-7
рН = – lg[H+] = 7
рОН = –lg[ОH-] = 7
pKw = pH + pOH = 14
рН is hydrogen ion exponent – a value that is equal to negative decimal logarithm of hydrogen ions concentration (mole/litre).
[H+] = [OH-] = 10-7
рН = – lg[H+] = 7
рОН = –lg[ОH-] = 7
pKw = pH + pOH = 14
рН is hydrogen ion exponent – a value that is equal to negative decimal logarithm of hydrogen ions concentration (mole/litre).
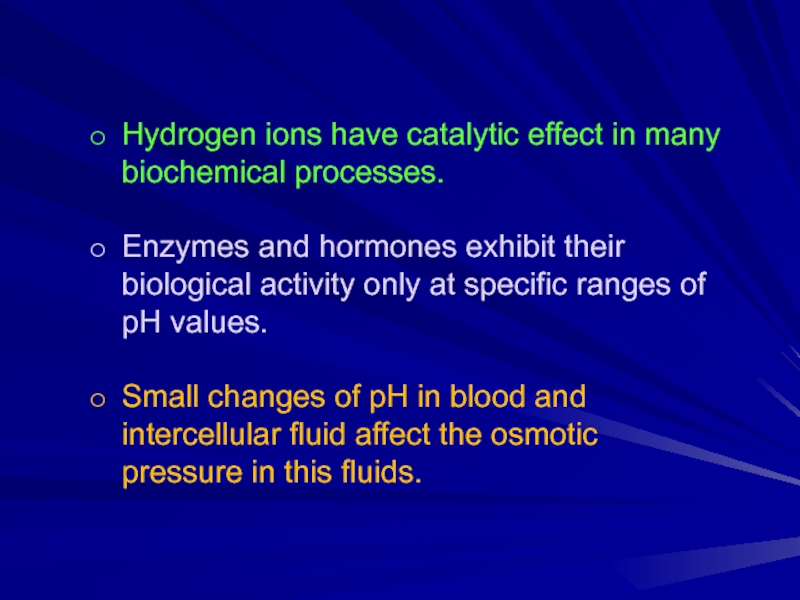
Слайд 6Hydrogen ions have catalytic effect in many biochemical processes.
Enzymes and hormones
exhibit their biological activity only at specific ranges of pH values.
Small changes of pH in blood and intercellular fluid affect the osmotic pressure in this fluids.
Small changes of pH in blood and intercellular fluid affect the osmotic pressure in this fluids.
Слайд 7BUFFER SOLUTIONS
are the solutions which pH values do not practically change
when moderate amounts of either a strong acid or a strong base are added and also as a result of dilution.
Buffer solutions consist of weak acids and their salts (conjugate bases) or of weak bases and their salts (conjugate acids).
Buffer solutions consist of weak acids and their salts (conjugate bases) or of weak bases and their salts (conjugate acids).
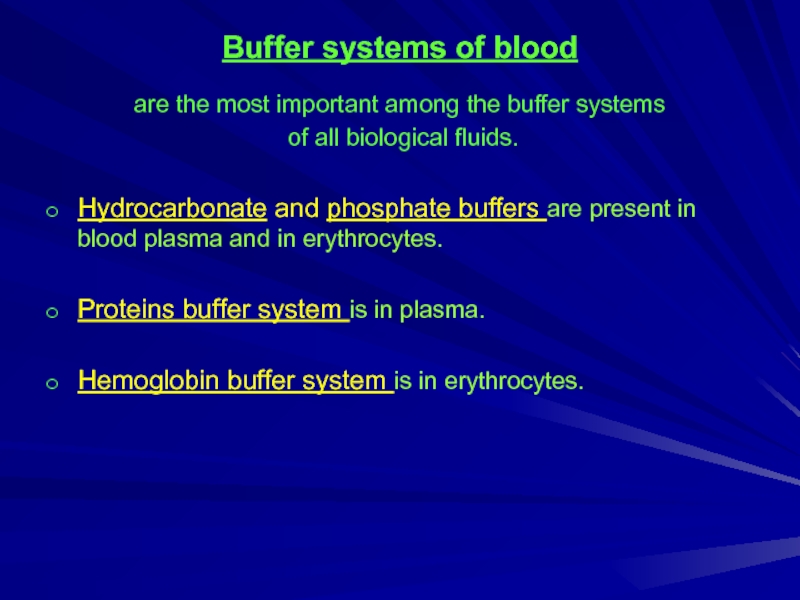
Слайд 8Buffer systems of blood
are the most important among the buffer systems
of all biological fluids.
Hydrocarbonate and phosphate buffers are present in blood plasma and in erythrocytes.
Proteins buffer system is in plasma.
Hemoglobin buffer system is in erythrocytes.
Hydrocarbonate and phosphate buffers are present in blood plasma and in erythrocytes.
Proteins buffer system is in plasma.
Hemoglobin buffer system is in erythrocytes.