- Главная
- Разное
- Дизайн
- Бизнес и предпринимательство
- Аналитика
- Образование
- Развлечения
- Красота и здоровье
- Финансы
- Государство
- Путешествия
- Спорт
- Недвижимость
- Армия
- Графика
- Культурология
- Еда и кулинария
- Лингвистика
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Детские презентации
- Информатика
- История
- Литература
- Маркетинг
- Математика
- Медицина
- Менеджмент
- Музыка
- МХК
- Немецкий язык
- ОБЖ
- Обществознание
- Окружающий мир
- Педагогика
- Русский язык
- Технология
- Физика
- Философия
- Химия
- Шаблоны, картинки для презентаций
- Экология
- Экономика
- Юриспруденция
Application of nickel nanoparticles in diffusion bonding of stainless steel surfaces презентация
Содержание
- 1. Application of nickel nanoparticles in diffusion bonding of stainless steel surfaces
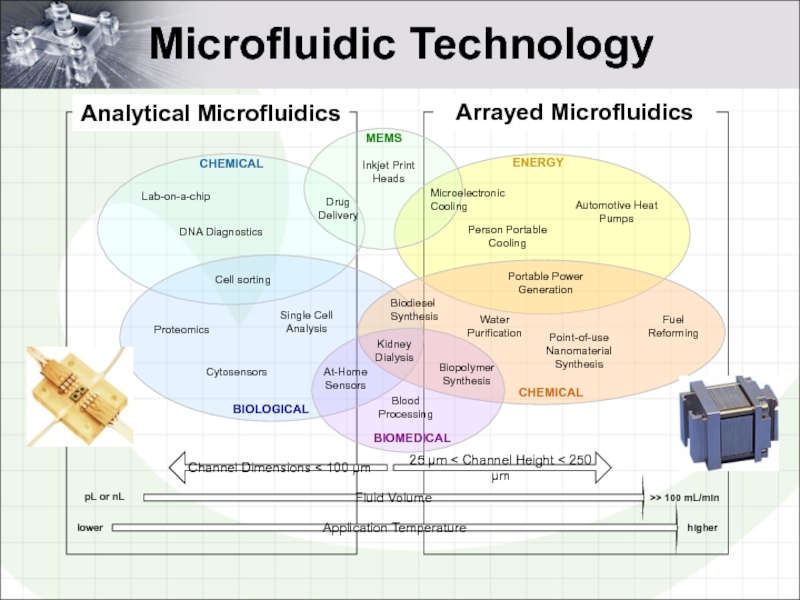
- 2. Microfluidic Technology Analytical Microfluidics Arrayed Microfluidics
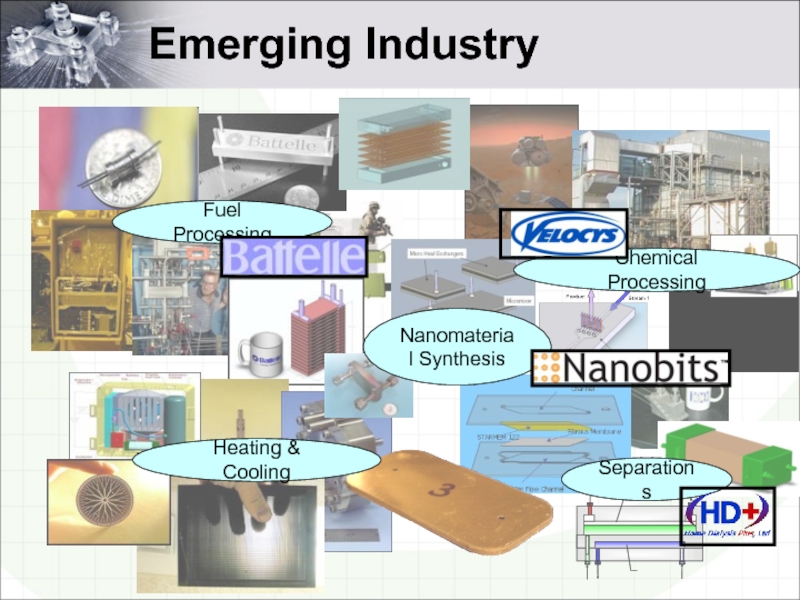
- 3. Emerging Industry Fuel Processing Chemical Processing Heating & Cooling Nanomaterial Synthesis Separations
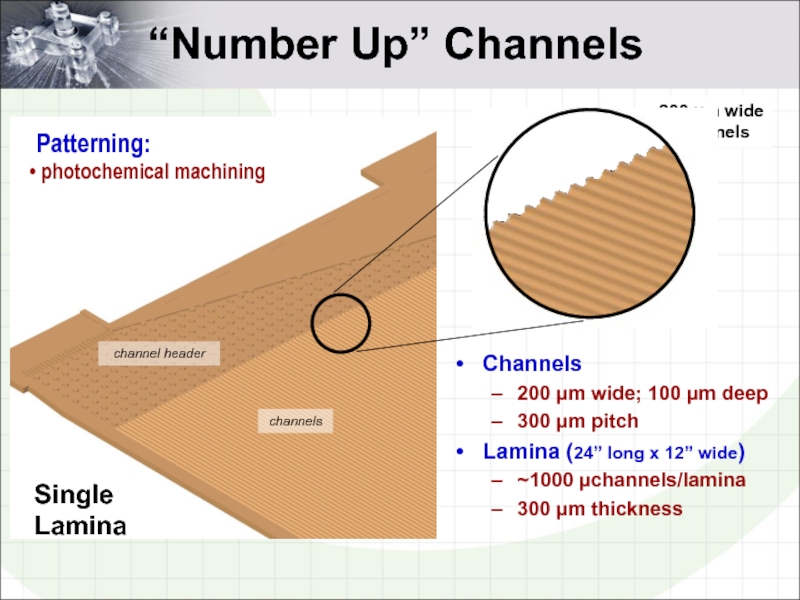
- 4. 200 µm wide channels “Number Up”
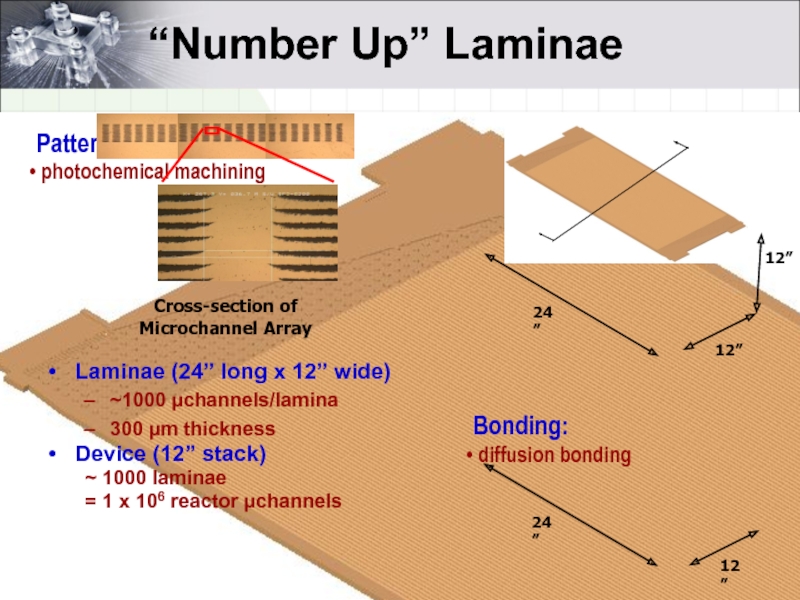
- 5. “Number Up” Laminae Device
- 6. Outline Motivation and Objective Approach Results Summary
- 7. Diffusion Bonding: Concept Initial 'point' contact
- 8. Diffusion Brazing of SS 316L Filler materials
- 9. Analysis of Microchannel Samples Objective To Compare
- 10. Scanning Electron Microscopy SEM image of bond
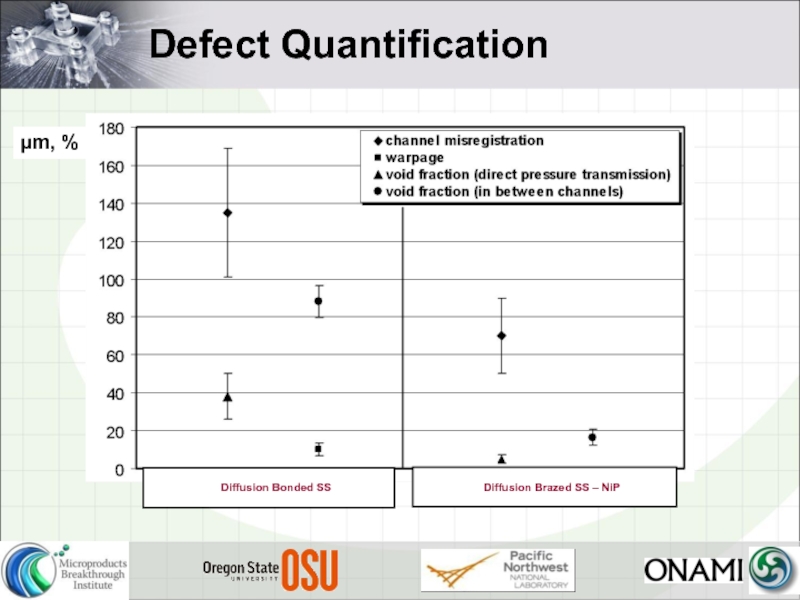
- 11. Defect Quantification µm, %
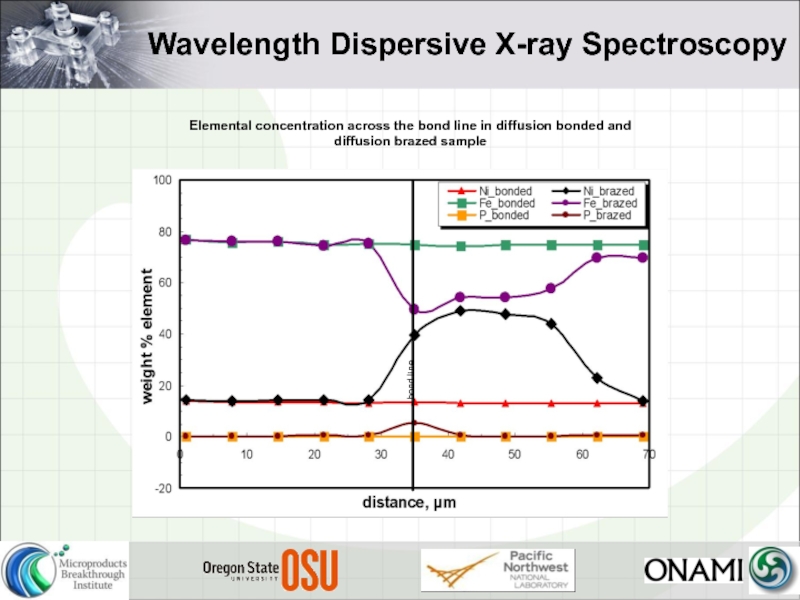
- 12. Wavelength Dispersive X-ray Spectroscopy
- 13. Nanoscale Materials in Chemistry, Wiley, 2001 Q
- 14. Role of Nanoparticles Nano-sized particles exhibit
- 15. Outline Motivation and Objective Approach Results Summary
- 16. Objective and Protocol Objectives to compare NiNP-brazed
- 17. Deposition from NP suspension Spin Coating
- 18. Nicrobraz Binder A commercially available water based
- 19. Film Characterization Continuous and uniform film Nanoparticle
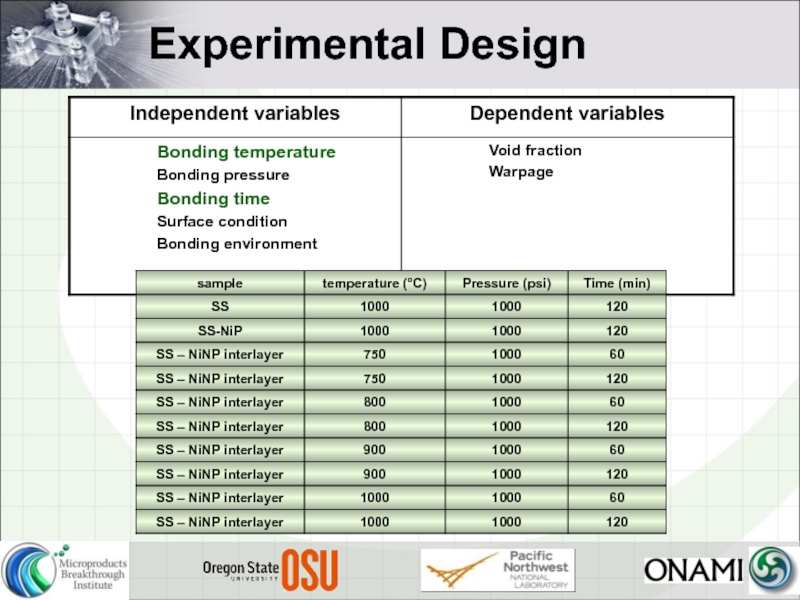
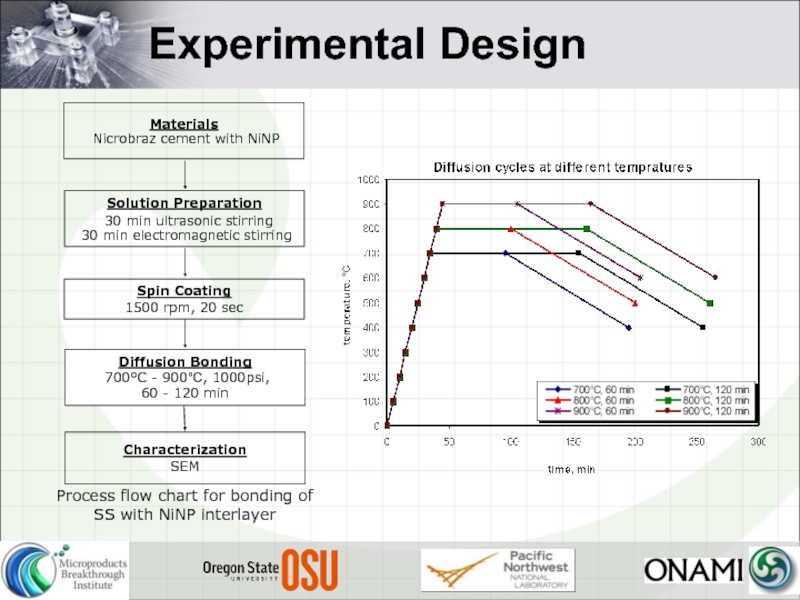
- 20. Experimental Design
- 21. Outline Motivation and Objective Approach Results Summary
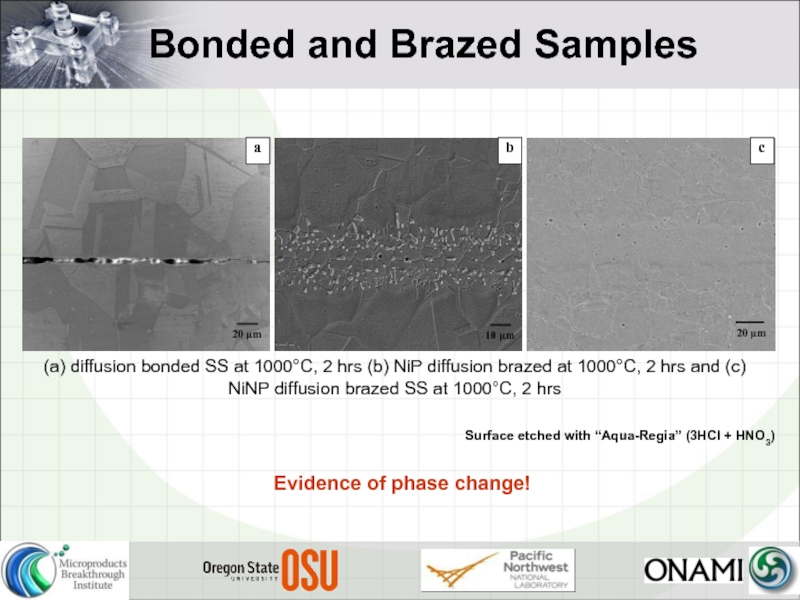
- 22. Bonded and Brazed Samples Surface etched with
- 23. Experimental Design Process flow chart for bonding of SS with NiNP interlayer
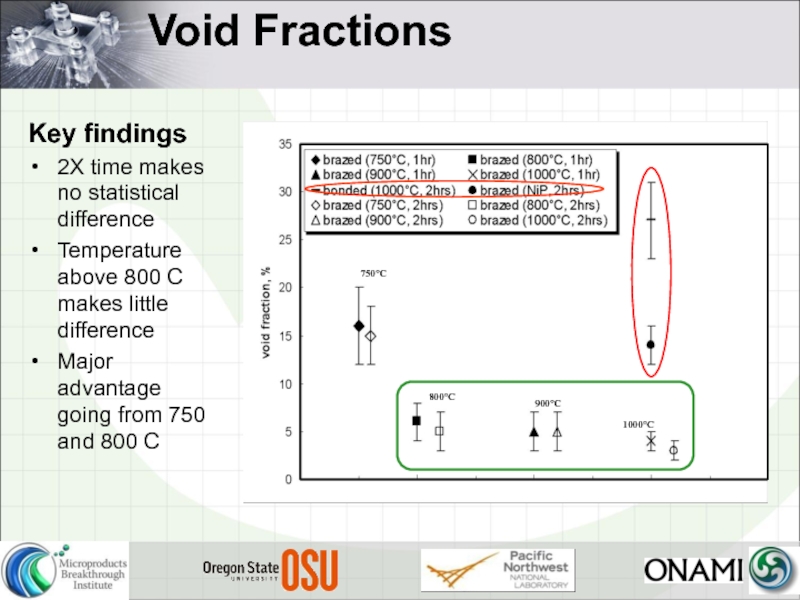
- 24. Void Fractions Key findings 2X time makes
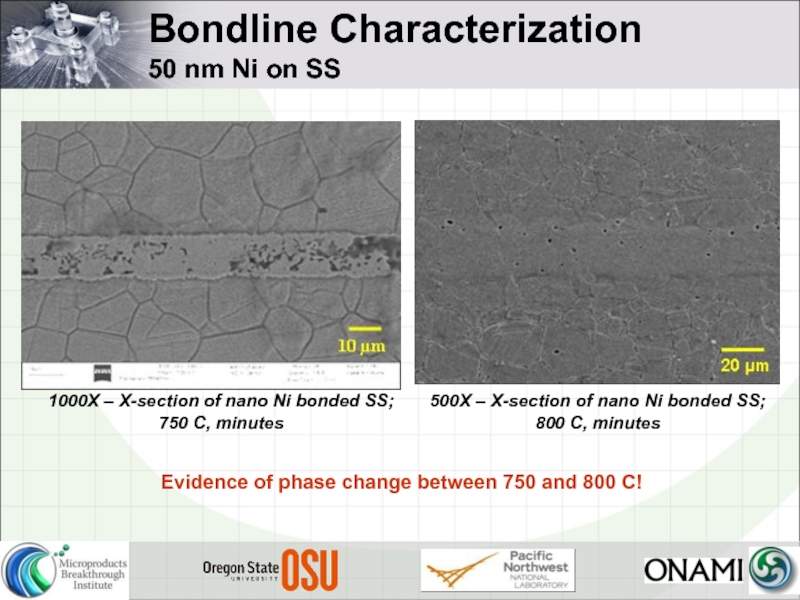
- 25. Bondline Characterization 50 nm Ni on
- 26. Summary A 50 nm+ dia. nickel nanoparticle
- 27. Acknowledgments This research is sponsored by the National Science Foundation CTS.
Слайд 1Application of Nickel Nanoparticles in Diffusion Bonding of Stainless Steel Surfaces
Santosh
School of Mechanical, Industrial and Manufacturing Engineering
Oregon State University
Слайд 3Emerging Industry
Fuel Processing
Chemical Processing
Heating & Cooling
Nanomaterial Synthesis
Separations
Слайд 4200 µm wide channels
“Number Up” Channels
channel header
channels
Single Lamina
Channels
200 µm wide;
300 µm pitch
Lamina (24” long x 12” wide)
~1000 µchannels/lamina
300 µm thickness
Patterning:
photochemical machining
Слайд 5“Number Up” Laminae
Device (12” stack)
~ 1000 laminae
= 1 x 106 reactor
Laminae (24” long x 12” wide)
~1000 µchannels/lamina
300 µm thickness
Bonding:
diffusion bonding
Patterning:
photochemical machining
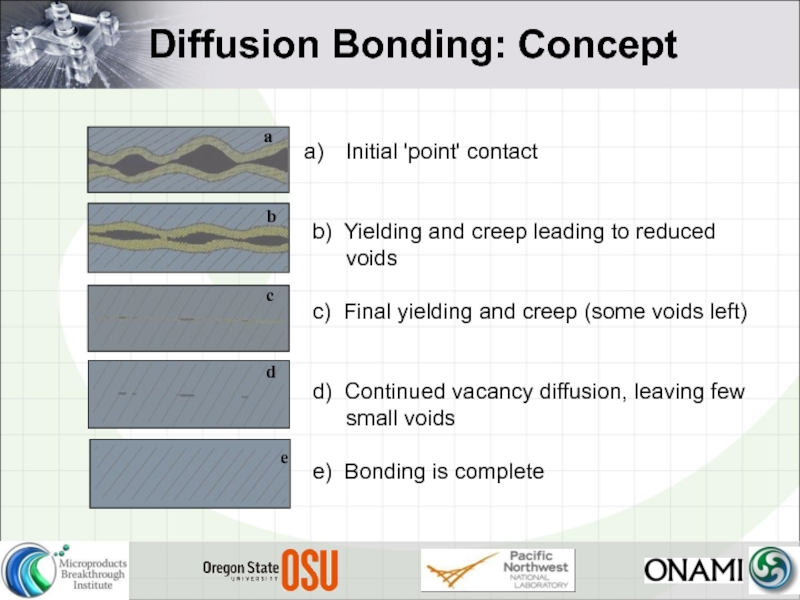
Слайд 7Diffusion Bonding: Concept
Initial 'point' contact
b) Yielding and creep leading to
c) Final yielding and creep (some voids left)
d) Continued vacancy diffusion, leaving few small voids
e) Bonding is complete
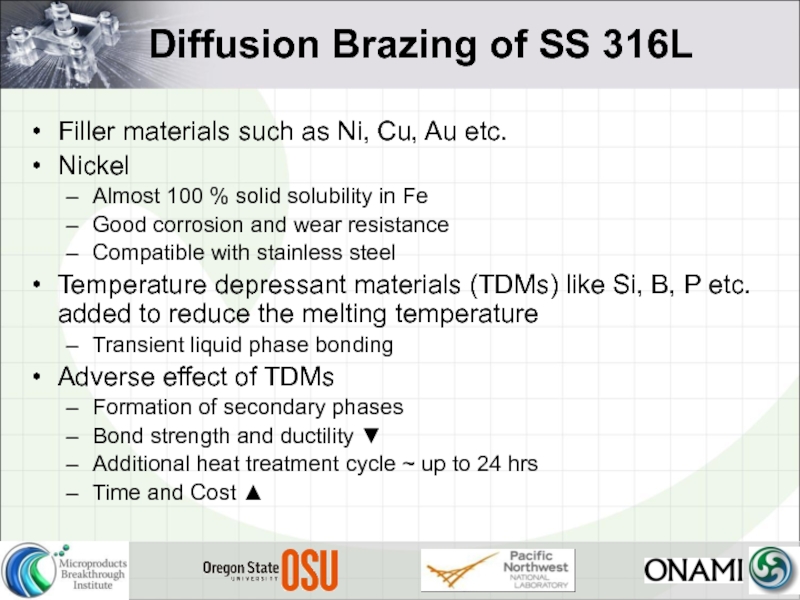
Слайд 8Diffusion Brazing of SS 316L
Filler materials such as Ni, Cu, Au
Nickel
Almost 100 % solid solubility in Fe
Good corrosion and wear resistance
Compatible with stainless steel
Temperature depressant materials (TDMs) like Si, B, P etc. added to reduce the melting temperature
Transient liquid phase bonding
Adverse effect of TDMs
Formation of secondary phases
Bond strength and ductility ▼
Additional heat treatment cycle ~ up to 24 hrs
Time and Cost ▲
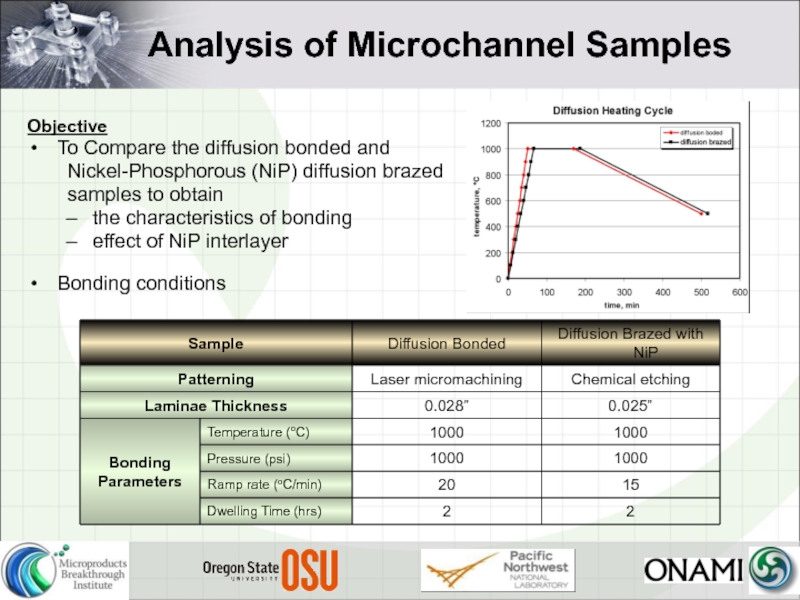
Слайд 9Analysis of Microchannel Samples
Objective
To Compare the diffusion bonded and
Nickel-Phosphorous (NiP)
samples to obtain
the characteristics of bonding
effect of NiP interlayer
Bonding conditions
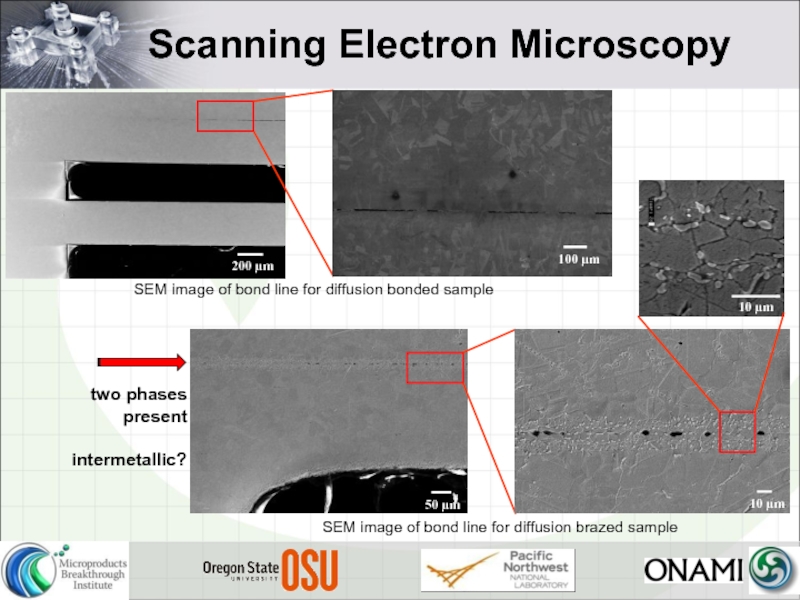
Слайд 10Scanning Electron Microscopy
SEM image of bond line for diffusion bonded sample
SEM
two phases present
intermetallic?
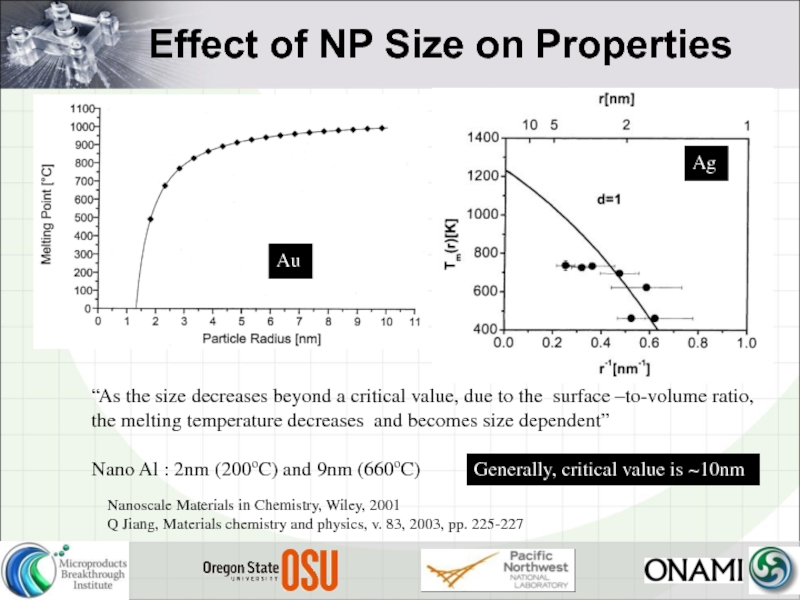
Слайд 13Nanoscale Materials in Chemistry, Wiley, 2001
Q Jiang, Materials chemistry and physics,
Au
Ag
“As the size decreases beyond a critical value, due to the surface –to-volume ratio, the melting temperature decreases and becomes size dependent”
Nano Al : 2nm (200oC) and 9nm (660oC)
Generally, critical value is ~10nm
Effect of NP Size on Properties
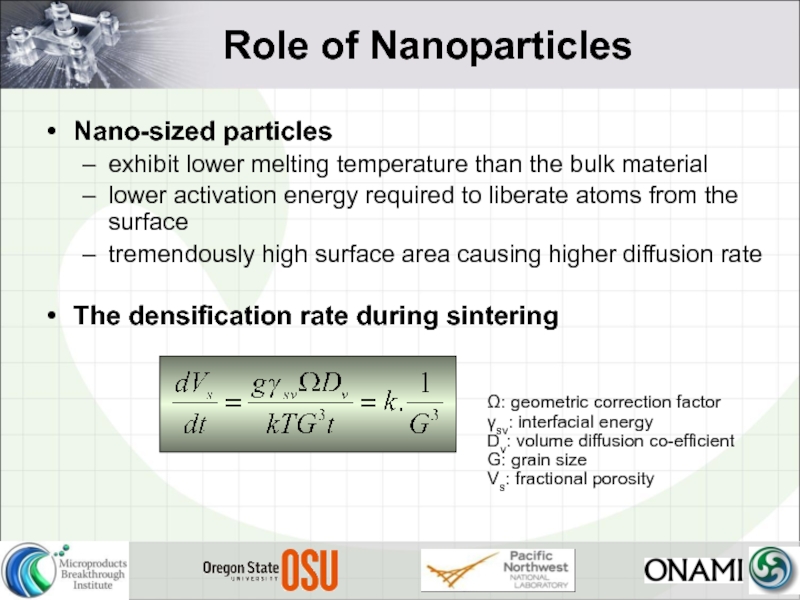
Слайд 14Role of Nanoparticles
Nano-sized particles
exhibit lower melting temperature than the bulk
lower activation energy required to liberate atoms from the surface
tremendously high surface area causing higher diffusion rate
The densification rate during sintering
Ω: geometric correction factor
γsv: interfacial energy
Dv: volume diffusion co-efficient
G: grain size
Vs: fractional porosity
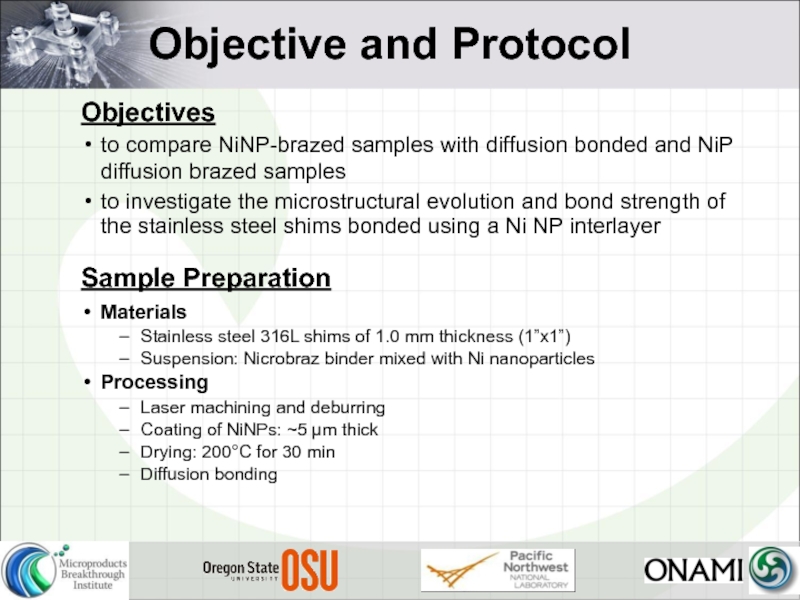
Слайд 16Objective and Protocol
Objectives
to compare NiNP-brazed samples with diffusion bonded and NiP
to investigate the microstructural evolution and bond strength of the stainless steel shims bonded using a Ni NP interlayer
Sample Preparation
Materials
Stainless steel 316L shims of 1.0 mm thickness (1”x1”)
Suspension: Nicrobraz binder mixed with Ni nanoparticles
Processing
Laser machining and deburring
Coating of NiNPs: ~5 µm thick
Drying: 200°C for 30 min
Diffusion bonding
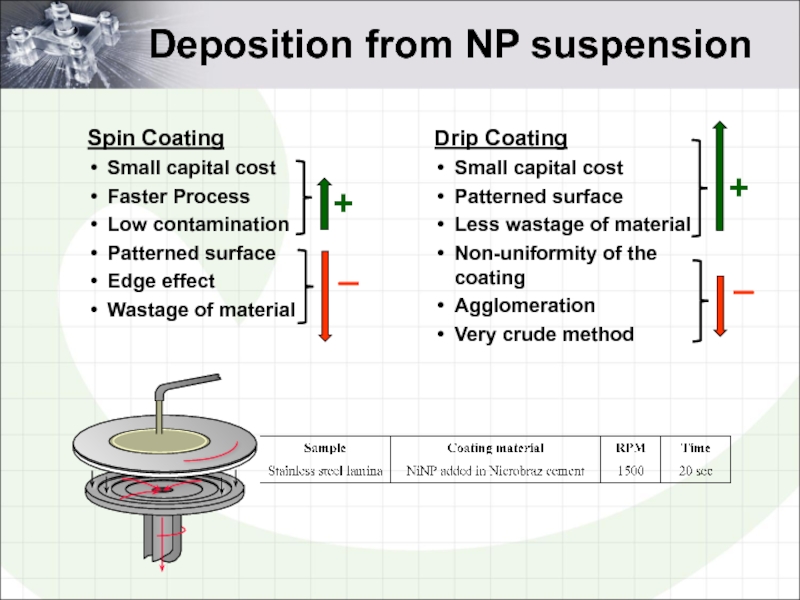
Слайд 17Deposition from NP suspension
Spin Coating
Small capital cost
Faster Process
Low contamination
Patterned
Edge effect
Wastage of material
Drip Coating
Small capital cost
Patterned surface
Less wastage of material
Non-uniformity of the coating
Agglomeration
Very crude method
Слайд 18Nicrobraz Binder
A commercially available water based binder (Wall Colmonoy Corporation)
Low viscosity:
Readily wets the surface of clean metal substrates
Excellent adherence and a relatively short drying time
Low content of binder material to minimize outgassing during the bonding cycle
All binding material volatilizes by 540°C leaving behind the compact layer of particles
No residue remains on the parts after brazing, when using nickel-based filler metals
Ideally suited for application of nickel-based brazing filler metals
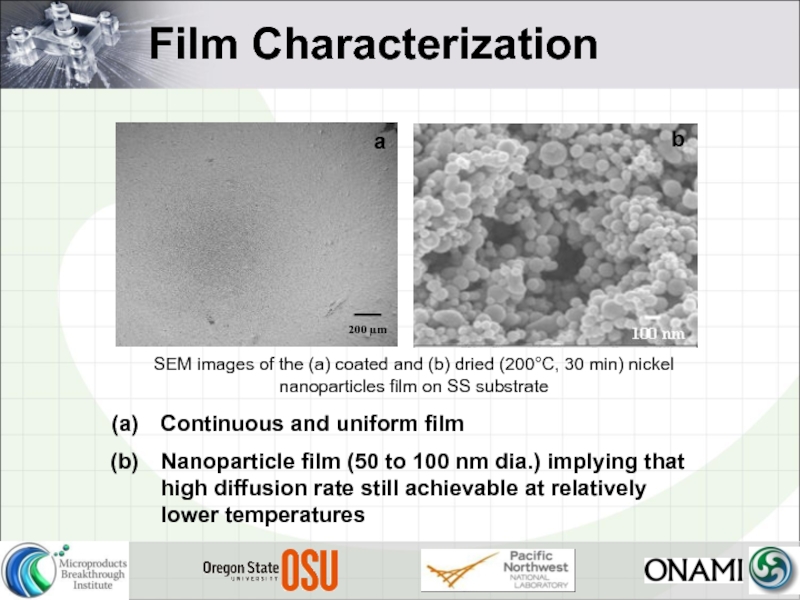
Слайд 19Film Characterization
Continuous and uniform film
Nanoparticle film (50 to 100 nm dia.)
SEM images of the (a) coated and (b) dried (200°C, 30 min) nickel nanoparticles film on SS substrate
a
b
Слайд 22Bonded and Brazed Samples
Surface etched with “Aqua-Regia” (3HCl + HNO3)
Evidence
Слайд 24Void Fractions
Key findings
2X time makes no statistical difference
Temperature above 800 C
Major advantage going from 750 and 800 C
Слайд 25Bondline Characterization
50 nm Ni on SS
1000X – X-section of nano
500X – X-section of nano Ni bonded SS; 800 C, minutes
Evidence of phase change between 750 and 800 C!
Слайд 26Summary
A 50 nm+ dia. nickel nanoparticle (NiNP) interlayer has been shown
lower the bonding temperature for diffusion brazing
eliminate the use of melting temperature depressants
NiNP-brazing yielded
low void fractions
no deleterious secondary phases
expected require less time at lower temperature than conventional diffusion techniques
50 nm+ dia. NiNPs appear to have gone through phase change between 750 and 800 C
Currently evaluating shear strength of joints